Coronavirus 2 of acute respiratory distress syndrome (SARS-CoV-2), the virus that caused the 2019 coronavirus pandemic (COVID-19), continues to spread and suppress. Scientists are working on a drug to control the virus. An exciting new study published on the bioRxiv * preprint server is describing a new platform that allows high-throughput workflow to generate human lung buds in the tens of thousands. This will allow researchers to study SARS-CoV-2 infection and treatment in lung tissue that mirrors the key aspects of human lung development.
Lung models required
Infection with SARS-CoV-2 can occur through the nose, or airways of the lungs, and alveoli. The virus appears to target the lungs, in particular, with severe lung injury and complications associated with pneumonia the most common complications of COVID-19 infection. The virus attacks the associated cells and type II pneumocytes of the lungs through the angiotensin-converting enzyme receptor 2 (ACE2) as well as other enzymes that are cofactors in viral entry.
However, the extent to which the virus damages lung tissue is not yet clear. Currently, the majority of studies on SARS-CoV-2 are performed in human primary lung tissue. However, these in vitro systems are invisible and the cells are widely variable, depending on the genetic and phenotypic profile of the tumor donor. These differences are significant in their impact on viral replication and clinical impact.
Another option of this is to use human pluripotent stem cells (hPSCs) into the airways of the lungs and alveolar cells, while other researchers have developed lung cells from hPSCs from organoids to molecules. identify and test small inhibitors of SARS-CoV-2. However, these models do not reflect the group of strains found in development and adult lungs, derived from primary signaling pathways that guide lung development. In addition, lung cells take 1-3 months to differentiate from hPSCs.
Synthetic human lung buds
The current study aimed to develop a platform to create lung-like material. These are generated from hPSCs, using their ability to self-organize when cultivated in a specific geometry on micropattern slits. They used special activators to generate thousands of lung progenitor cells, arranged in colonies, on a single micropattern chip.
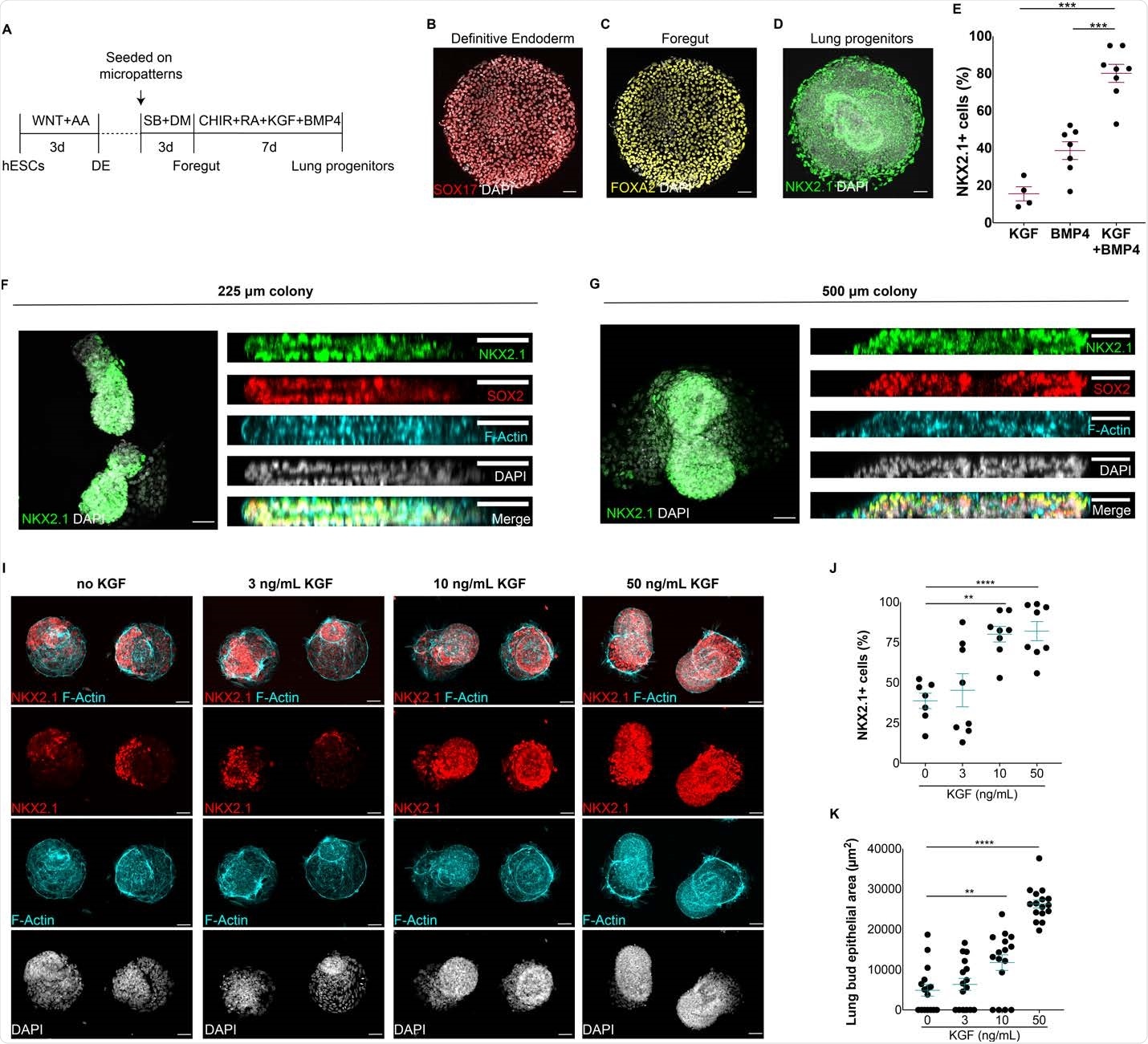
Formation of autologous epithelial lung buds on finite geometry. A) Protocol for the generation of synthetic lung buds on finite geometry in micropatterns. BD) SOX17 + (B) endoderm cells, FOXA2 + anterior endoderm cells (C) and NKX2.1 + multipotent lung progenitors (D) were generated at the end of definite endoderm (DE), foregut and lung progenitor entry levels, respectively. E) Extract of NKX2.1 + lung enhancers when modified by BMP4, KGF or BMP4 / KGF for 7 days. FG) Topical and lateral views of 3D epithelial fissures containing multicellular NKX2.1 + lung promoters in 225 and 500 μm colonies. I) Micropattern colonies containing NKX2.1 + epithelial buds at increasing doses of KGF. J) Proportion of NKX2.1 + progenitor cells at increasing doses of KGF. K) Enlargement of lung bud epithelial area in micropattern colonies at different doses of KGF. (** p <0.01, *** p <.001, **** p <0.0001, Dunnett multivariate test; scale bar, 50 μm)
They found that not only did these synthetic human lung tumors exhibit early differentiation parameters, but they adapted themselves to become human lung buds. They were also able to discover some crucial steps in early differentiation and discover novel relationships among alveolar cells.
They found that the virus affects these lung tumors, both connected cells, and type II alveolar cells. The latter are more likely because they have a higher level of ACE2 and TMPRSS2, as well as a sense of furin, and are all essential for SARS-CoV-2 infection. They also found suggestions that alveolar cell division is also more vulnerable to the virus.
The researchers measured infection at 48 and 96 hours from the start and found that infectious cells proliferated over time in all cell types, suggesting that viral transmission occurs within lung buds. synthetic human. They also found apoptosis occurring in alveolar and airway cells. So they could use a single micropattern chip growing hundreds of synthetic human lung cells to monitor for infection vulnerability, identify susceptible cell types, assess viral transmission between cells, demonstrate cytopathic effects in hundreds of synthetic lung germs on it and so the entire viral life cycle continued.
They then screened for chemotherapy that had demonstrated in vitro efficacy in binding assays and in cell lines. They tested the neutralization of antibodies in human convalescent plasma, for their effectiveness in preventing infection across a range of dilutions. They found that the results were comparable to those obtained using cell lines. Again, they found that the neutralizing antibodies could also prevent the spread of the virus through the organoid even after the infection has occurred. This supports the potential of these drugs as a therapeutic, acting as biological inhibitors of SARS-CoV-2 infection and cell-to-cell transmission.
What is the impact?
Scientists have not only shown that lung stem cells can self-organize when cultivated in finite geometry but that these organs repeat the same path in which they do. fetal lung promoters undergo lung development. This could be of great benefit in research into lung disease and regenerative medicine involving models of lung by providing fast and unlimited access to human organoid lung tissue that has genetically matched.
Differences in disease vulnerability between different cells, and differences in the viral effect on different cell types, can be quantitatively studied using this platform. In fact, this model allowed scientists to identify some cell types that are difficult to study. It may also lead to a better understanding of why SARS-CoV-2 is more pathogenic than other coronaviruses.
Moreover, these findings reveal new types of cells and pathways of cell development in fetal life, which may help to understand how human lungs differ. This could also shed light on the origins of lung cancer and other diseases.
“We anticipate that our synthetic human lung model will be transformative for clarifying the molecular basis of currently untreated respiratory and lung diseases.. This study also highlights the potential use of synthetic lungs to identify therapeutic treatment that prevents infection with SARS-CoV-2, endemic coronaviruses as well as other respiratory viruses.. ”
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.