A team of scientists from Sweden and India have recently identified the host cell metabolic changes associated with severe respiratory disease coronavirus syndrome 2 (SARS-CoV-2). Their findings suggest that SARS-CoV-2 alters the central carbon metabolism of the host cell to enable reproduction and multiplication of infections. In addition, they claim that the severity of the 2019 coronavirus infection (COVID-19) is largely dependent on blood levels of glucose, mannose, and glutamate. The study is currently available on the bioRxiv* preprint server.
Background
Since its emergence in late December 2019 in Wuhan, China, SARS-CoV-2, the causative pathogen of COVID-19 infection, has captured 114 million people and claimed 2.52 million lives worldwide.
Although 80% of COVID-19 patients remain asymptomatic or slightly symptomatic, the risk of developing severe disease is higher among people with metabolic comorbidities, such as diabetes and obesity. In addition, there is evidence to show that COVID-19 depletion is associated with a number of metabolic changes, including increased amino acid and fatty acid and altered lipid and energy metabolism.
In terms of the viral life cycle, glucose and glutamine are known to be required as extracellular carbon sources for viral reproduction and that a virus is able to alter several metabolic pathways of host cells, including central carbon metabolism , to enable his life- circle.
Regarding SARS-CoV-2 infection, studies have shown that targeting glycolysis and PI3K / AKT signaling pathways by small molecular protectors can lead to a significant reduction in viral loading in infectious cells.
In the present study, scientists have examined the involvement of major host cell metabolic pathways in SARS-CoV-2 reproduction. They have also investigated whether changes in host cell metabolic profiles are associated with COVID-19 depletion.
Study design
The scientists analyzed 92 inflammatory mediators in plasma using a targeted proteomics approach. In addition, they performed plasma metabolic profiles using unspecified metabolomics, followed by phenotyping of lymphocyte and monocyte immunity towards metabolite transporters.
In a separate set of in vitro experiments, performed noninvasive quantitative proteomics using lung, liver, kidney and colon SARS-CoV-2 cells to understand mediated metabolic remodeling.
Important comments
The proteomics and metabolomics analyzes were performed using plasma samples collected from hospitalized COVID-19 patients with moderate or severe form of the disease. The targeted proteomics data revealed significantly increased cytokines and chemokines in mild and severe COVID-19 patients. Interestingly, the scientists observed a lower level of interleukin 12 (IL-12) in severe COVID-19 patients compared to that in patients with few effects.
444 significantly altered metabolites were identified in COVID-19 patients using undifferentiated plasma metabolomics. Most of these metabolites were lipids and amino acids. With further analysis, the scientists found that SARS-CoV-2 pathways significantly affect amino acid metabolism pathways. Interestingly, they found that the levels of glycolysis and metabolites associated with the TCA cycle varied significantly between COVID-19 patients with diverse disease complications.
While comparing mild and severe COVID-19 patients, the scientists found that different amino acid-related pathways, insulin signaling pathways, and macrophage-mediated IL production and signaling were significantly affected. -12 in severe patients. They identified plasma levels of glucose, mannose, and glutamate as the main determinants of disease severity by further analysis. Although they observed an elevated plasma level of mannose-binding lectin in COVID-19 patients, there was no association between mannose and mannose-binding lectin levels.
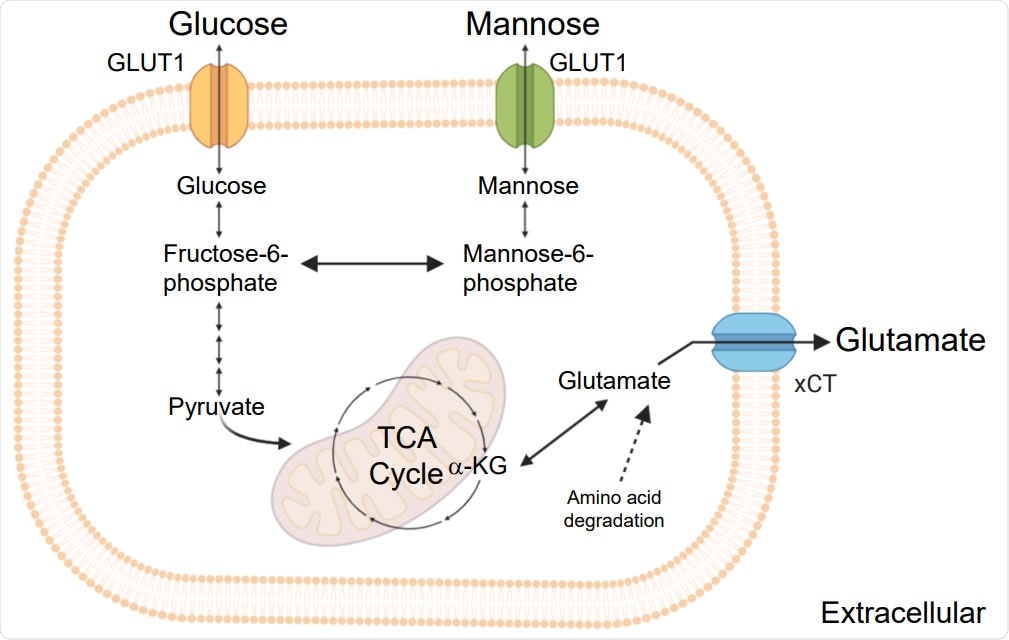
Decorticated production of the main stages of glycolysis, mannose and glutamate metabolism and TCA cycle.
Because the metabolite transporters can regulate immune cells, such as lymphocytes and monocytes, by controlling nutrient supply, the scientists performed immune phenotyping of glucose, mannose, and glutamate transporters. They observed significantly reduced lymphocyte levels, elevated moderately high monocyte levels, and markedly decreased nonclassical monocyte levels in COVID-19 patients. These observations reveal a significant involvement of monocytes in altered immune responses during SARS-CoV-2 infection. Interestingly, they observed very high expressions of metabolite transporters in all subpopulations of monocytes studied.
The in vitro Tests performed to assess host cell metabolic changes in response to SARS-CoV-2 disease showed that proteins involved in glycolysis / gluconeogenesis and fructose and mannose metabolism were significantly increased in lung cells but were significantly reduced mitochondrial TCA ring proteins. This indicates that SARS-CoV-2 infection may cause mitochondrial dysfunction.
In addition to glucose and glutamate, they saw elevated levels of pyruvate, lactate and α-ketoglutarate, indicating a significant involvement in glycolysis and glutaminolysis pathways in SARS-CoV-2 disease. With these decisions, they blocked these pathways and saw a significant reduction in viral reproduction. By altering the amount of glucose and mannose in culture media containing cells with SARS-CoV-2 infection, they found that an increase in glucose levels has an effect on viral reproduction.
Investigate meaning
The study shows that, to facilitate reproduction, SARS-CoV-2 alters central cell carbon metabolism, converting carbohydrates into a precursor for metabolism. In addition, the study identifies metabolites of the carbohydrate and amino acid metabolism pathways as potential biomarkers for predicting the severity of COVID-19.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.