.jpg)
A new study finds that saliva testing has a high sensitivity for detecting acute respiratory syndrome 2 (SARS-CoV-2) in symptomatic and asymptomatic patients.
Routine diagnostic tests rely on nasopharyngeal tests where they swim for viruses such as SARS-CoV-2 that cause respiratory infections. But collecting viral samples can be an uncomfortable experience, which can prevent people from getting tested. There is also a risk of exposure for healthcare workers who carry out the tests.
Andrew C. Nelson of the University of Minnesota and colleagues suggests that a self-assembled saliva test is another method that can be used for nasal swabs. People who collect their own saliva samples would be assisted by extensive testing efforts. It would also reduce the risk of healthcare workers appearing when performing these diagnostic tests.
The authors write:
“Understanding how anatomic location, collection time in the course of the disease, treatment and transport of the sample, and analysis platform may influence test results is crucial for making informed medical decisions regarding the administration of COVID-19… Overall, our findings support the conclusion that self-collected saline testing is effective for the detection of COVID-19, especially at early stages of disease progression. “
The study “An accurate salivary test for early and presymptomatic COVID-19” is available as a preview of the medRxiv* server, while the article is under peer review.
Collecting test samples
The team looked for significant differences between saliva samples collected from patients and nasal swabs.
Thirty nasopharyngeal test samples were collected from several peers in both patient and patient setting.
In the first group were 354 patients who underwent routine clinical trials for symptomatic or asymptomatic concerns of SARS-CoV-2. Approximately 80% of cases were symbolic of the group. Thirty negative cases for SARS-CoV-2 were randomized for comparison purposes.
Cohort 1 was further divided into two groups based on how samples were evaluated. Cohort 1A included outpatient trials used in hospitals, and Cohort 1B was subjected to nasopharyngeal testing with commercial testing platforms.
In a second group, the researchers obtained nasal, nasopharyngeal, and salivary samples from hospitalized patients who tested positive for COVID-19. All samples were obtained 48 hours upon admission to the hospital.
The nasopharyngeal samples were collected by a healthcare professional, while saliva and nasal samples were previously collected only under the guidance of the researchers.
To monitor for COVID-19-related symptoms, the researchers reviewed medical records to report examples of loss of taste or smell, shortness of breath, cough, sore throat, muscle weakness, wheezing. diarrhea, nausea or vomiting, loss of appetite, chest pain, or signs of a headache.
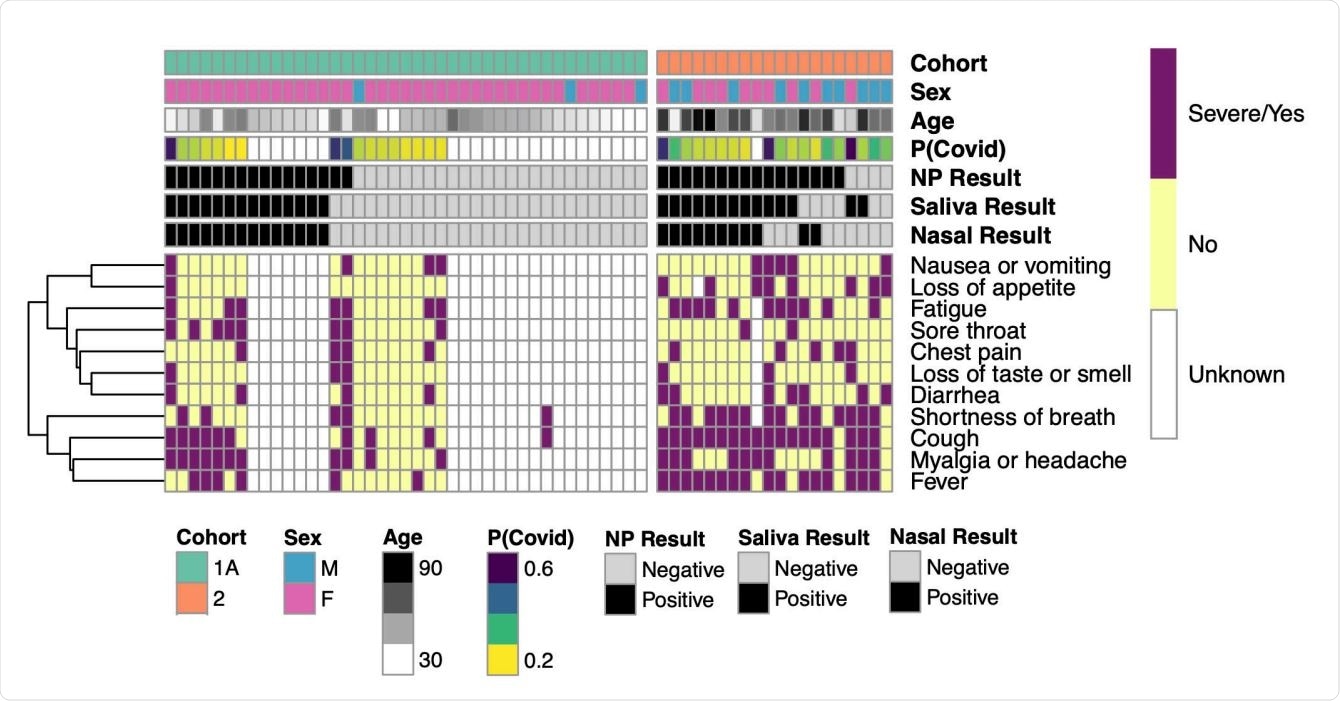
Symbolic heat map for agreement between positive and negative test results by test method and cohort. Participants have heat map columns and there are rows of signals. Symbolic presence is marked as either solid or yes (purple), no (yellow), or unknown (white). The signals are collected by appearance and a collection is associated with the dendrogram (left). Test results are marked by color as either positive (black) or negative (light gray). The computed probability of each participant of COVID-19 (P (COVID)) is characterized by higher probabilities shown in purple and lower probabilities in yellow. Partner gender, age, and cohort are also identified.
Variability between diagnostic tests
In Cohort 1A, the nasopharyngeal samples as a whole showed 16 positive cases and 25 negative cases. Results from salivary and nasal samples were also 100% inaccurate in detecting these cases.
In Cohort 1B, the nasopharyngeal samples showed 14 positive cases and 5 negative cases. However, the commercial diagnostic tests evaluating nasal samples and saliva showed lower sensitivity at 57.1% compared to nasopharyngeal clinical trials. There was also a difference between nasal and salivary samples showing 87.5% sensitivity and 89.5% overall percentage agreement.
The following COVID-19 positive cases at Cohort 2:16 were detected by nasopharyngeal test, 14 were detected by saliva test, and 11 by nasal samples. Across all diagnostic tests, 69% –82% detected sensitivity and 65% –75% overall percentage agreement.
Clinical sensitivity in Cohort 2 was highest with nasopharyngeal samples at 89%, while salivary tests were sensitive at 78%. Of the three diagnostic tests, nasal samples were worst at 61%.
Salivary samples identified two COVID-19 patients who had received negative tests from nasopharyngeal tests on the same day.
Cohort 2 had the greatest variability for viral loads when researchers analyzed bicycle threshold data points. They found areas of viral loading across all anatomic sites with values as low as 26.2 at later disease stages.
Sensitive saliva test in the early stages of the disease
After performing a medical card examination, they calculated initial symptoms and assigned P scores (COVID) based on a previous prediction model.
They found that salivary test was more sensitive than nasopharyngeal samples in detecting SARS-CoV-2 when the onset of symptoms was still early. The findings state that “salivary and nasal samples perform well in a population screening setting.”
However, the researchers noted that P (COVID) scores were more biased toward the symptom of loss of taste or smell, which was topically assessed by the patient.
* Important message
medRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical / behavioral practice, or be treated as information established.