Coronaviruses can infect both animals and humans. Coronavirus infections are common, and have some zoonotic strains, meaning that they can be passed between animals and humans.
The true respiratory coronavirus 2 (SARS-CoV-2) syndrome, the virus that causes the coronavirus infection (COVID-19), appeared in Wuhan City, China, in late December 2019. Although the real reservoir animals of the virus yet to be identified, scientists believe the virus originated from a horse bat and jumped to intermediate hosts before making its way to the human population.
Researchers at the Beijing Center for Computer Science Research and Peking University analyzed sequences of the angiotensin-converting enzyme 2 (ACE2) proteins from 16 mammals and predicted the structures of ACE2- receptor-binding domain (RBD) .
The team has found that the ACE2 proteins of cow, cat, and panda form a strong link to the RBD. Meanwhile, the ACE2 proteins of rats, bat, horse, horse, mouse, and civet interact weakly with RBD.
The study suggests that those animals with strong ACE2 binding to RBD are more likely to be infected or host of the virus.
Zoonotic disease
Over the past few years, a number of zoonotic events have occurred across the globe. These include zoonotic flu, such as H1N1 or swine flu, salmonellosis, West Nile virus, plague, rabies, Lyme disease, and emerging coronaviruses, such as severe respiratory syndrome (SARS), Middle Eastern respiratory syndrome (MERS), and SARS-CoV-2, among others.
Some coronaviruses cause cold-like illnesses in humans, while others cause illness in some animals, such as cattle, bats, and camels. At the same time, some coronaviruses, such as feline and canine coronary viruses, only affect animals. However, zoonotic outbreaks can occur, just as in the pandemic of coronavirus, which has now spread to 192 countries and regions. To date, there are over 104 million cases of COVID-19 worldwide. More than 2.25 people have died from the disease.
The study
The current review, published on the pre-print server bioRxiv *, to identify potential host animals for SARS-CoV-2 to help prevent future outbreaks.
ACE2 is a protein on the surface of many cell types. It is an enzyme that generates small proteins by breaking up the major angiotensinogen protein, which in turn regulates cell activity.
Using the spike-like protein on its surface, the SARS-CoV-2 virus binds to ACE2. As soon as the spike protein, especially the RBD, binds to the ACE2, the virus can enter the human cell and cause infection.
Because ACE2 is widely present in mammals, it is essential to study the interactions of RBD and ACE2 of other mammals.
To reach the conclusions of the study, the researchers analyzed sequences of ACE2 proteins from 16 mammals and predicted ACE2-RBD structures. The team studied the order, structure and dynamics of these centers, giving us valuable insight into the interaction between ACE2 and RBD.
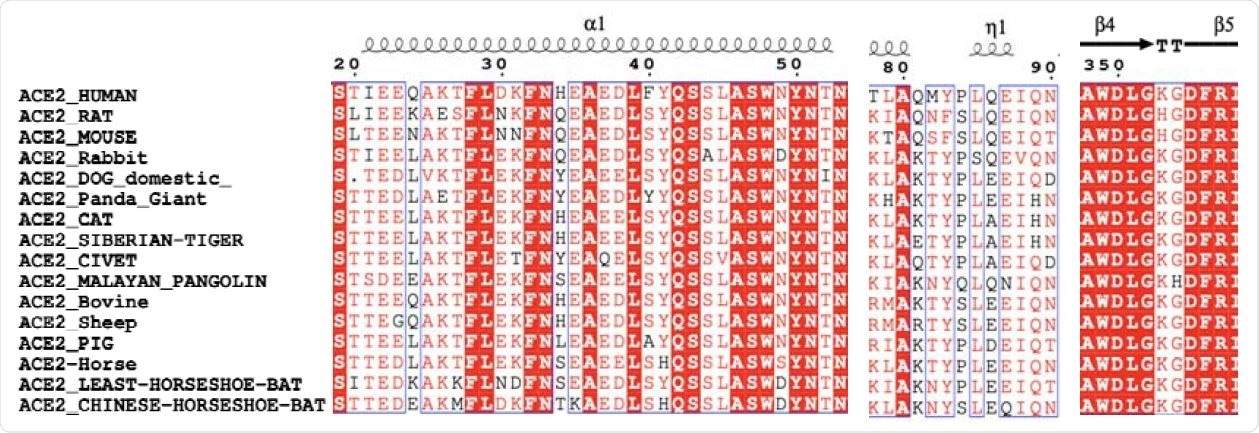
Comparison for the main residues of the binding interface facilities after sequence alignment analysis.
The ACE2 sources selected in the study included humans, cattle or cow, cat, Chinese horse bat, dog, giant panda, horse, lesser horse bat, Malayan pangolin, mouse, palm civet, pig, rabbit, rat, sheep, and the Siberian tiger.
The team obtained the ACE2 sequences from the NCBI and uniport databases. They would form structures for 15 mammalian ACE2 proteins. The SARS-CoV-2 RBD and ACE2 substitutes were then collected.
The team calculated and visualized the electrostatic potential maps at the complex ACE2-RBD interface. At the same time, they also performed molecular dynamics simulations of ACE2-RBD ratios.
The results of the study showed that cats, pandas, cattle or cattle, and humans form a strong interaction with the RBD, while the ACE2-RBD is weaker in dogs, Siberian tigers, Malayan pangolins, sheep , and rabbits. In mice, civets, horses, rats, pigs, and lesser horse bats, the interaction was much weaker. Further, the ACE2 of cattle and sheep exhibits high sequence identity to human ACE2.
“This study provides a molecular basis for different interactions between ACE2 and RBD in 16 mammals and will be useful in predicting the host range of the SARS-CoV-2,” he concluded. the authors of the study. Knowing the host area of the novel coronavirus can help predict and prevent future outbreaks.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.
Magazine Reference:
- Lupala, C., Kumar, V., Su, X., and Liu, H. (2021). Computational observations of differential interaction of mammalian ACE2 with SARS-CoV-2 spike receptor binding domain. bioRxiv. https://doi.org/10.1101/2021.02.02.429327,