The main thrust of a scientific study of therapeutic therapy to combat chronic coronavirus disease 2019 (COVID-19) has focused on identifying drugs and antibodies that may counteract the entry of the causative virus , severe respiratory syndrome coronavirus 2 (SARS-CoV -2), into the host cell.
A new study by US-based researchers shows that there are protective antibodies that target the N-terminal domain (NTD) of the spike instead of the spike-binding domain (RBD).
The team – from Vanderbilt Vaccination Center, Washington University School of Medicine, St. Louis, and Integral Molecular – introduced bioRxiv * server.
Protein spike
Viral entry is mediated by the virus’s spike protein, which binds to the host cell receptor, angiotensin-converting enzyme 2 (ACE2), through RBD. The spike is a trimeric glycoprotein with two subunits for each protomer, the S1 and the S2. The RBD is in the S1, and is responsible for binding the ACE2.
The S2 subunit mediates viral cell membrane fusion and viral entry into the cell. So most neutralizing antibodies have targeted the RBD spike.
The RNA genome and protein transcriptome of the virus have altered thousands of times, with an average of four mutations per amino acid in the spike protein. This has raised concerns about whether viral changes would allow the escape of neutralizing antibodies, most of which bind to the RBD. Some scientists have reported monoclonal antibodies (mAbs) that bind to regions outside the RBD, too.
Neutral activity
The current study identifies the structure and viability of three anti-NTD antibodies selected from a library of approximately 390 mAbs obtained from individuals who had recovered from natural SARS-CoV-2 infection. Two of the three had powerful neutral ability. These were named as COV2-2676 and COV2-2489.
They found, in two types of neutralization assays, a focal reduction neuroimaging test (FRNT) using the live virus, and a second real-time cell analysis (RTCA) test that used the reversible vesicular stomatitis virus. spike-expressing (capable) (VSV) pseudovirus. They found that both mAbs were able to neutralize the wildtype virus in the FRNT in a dose-dependent manner, with the half-maximum inhibitory value (IC50) at 501 or 199 ng / mL, separately. In the latter, the two neutralized the pseudovirus with IC50 at 8 or 56 ng / mL, respectively.
Common binding division
The scientists identified the epitopes that bound these neutral antibodies. Both bound to a specific antigenic site compared to the epitopes bound to human anti-RBD mAbs. Both of these antibodies failed to inhibit ACE2-soluble RBD binding. In fact, both antibodies are attached to the NTD of the spike in the ‘closed’ concordance of the spike trimer. They compete fiercely with each other because of the common binding epitope. Both are independent clonotypes, coded by the heavy-chain variable gene segment IGHV-4–39 and IGHV1–69, respectively. Their HCDR3 regions are quite different, however.
SARS-CoV-2 NTD spike identification
The heavy loops of these two antibodies interact with the NTD spike at the loops N3 and N5, respectively, indicating that this is a potential therapeutic target, and that individuals with immunity to the virus secreting antibodies targeting this region.
Using alanine scans, the researchers also concluded that single alanine modifications at several positions involved in the binding of these mAbs did not alter binding to another anti-NTD antibody, COV2-2305, possibly because these loops have the main communication remnants in strategic locations. . Using the RTCA assay, they detected escape mutations that counteracted neutralization with these antibodies at 10 or 100 μg / mL levels of COV2-2676 and COV2-2489, respectively. The escape mutations were in the same area identified by the alanine scan. Thus, both methods indicate a common antigenic site of binding for both antibodies, with different epitopes within this region.
Attaching spike to infectious cells
They found that these mAbs were strongly associated with protein spoilage on the surface of infected cells, with greater efficacy and binding density compared to antibodies targeting the RBD. They repeated the FRNT on several cell lines infected with the virus. They found, however, that this association varies according to cell type, possibly because the entry and receptor factors for the virus differ between different cell types.
They also compared the results of ingesting the mAbs with the virus before and after it was trapped on the cell surface. They found that the use of these antibodies resulted in strong neutralization both before and after the virus. These findings suggest that they could be used to prevent infection even after the virus has infected the cell.
Neutralization was not observed with these mAbs in cells that overexpressed human ACE2 and TMPRSS2, although anti-RBD antibodies inhibited viral binding.
Full length or F (ab ‘) 2 forms required
Both of these mAbs were able to neutralize the virus only in the form of full – length IgG or divalent Fab, but the monovalent Fab particles were unable to neutralize either. In contrast, Fabs of strongly neutralized anti-RBD antibodies prevented infection as effectively as the full-length antibody.
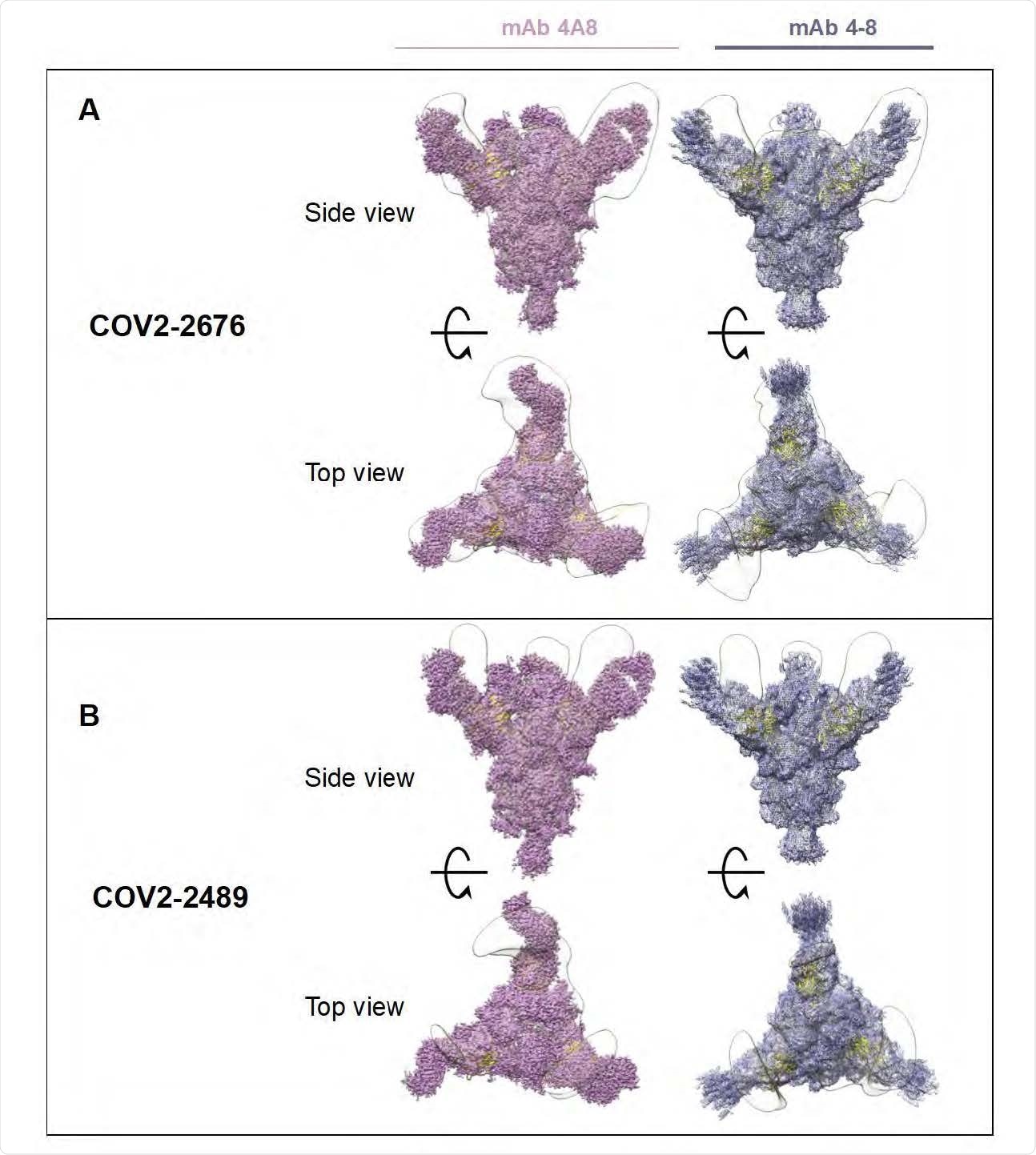
Fab-Spike EM negative negative style. A) COV2-2676 with mAb at the top has a side view of 4A8 (left), mAb 4-8 (right) and at the bottom has a top view of the same. B) COV2-2489 with mAb on the top has side view 4A8 (left), mAb 4-8 (right) and at the bottom has the top view of the same.
The researchers speculate, “The greater IgG or F (ab ‘) 2 could inhibit the functional interaction of the S protein in induction. On the other hand, the monovalent Fab molecule may bind with too low a relationship to the NTD to prevent entry. ”
Centralized equipment
These antibodies required complete Fc-mediated actions for their activation in vivo. When these interactions were eliminated, the mice developed more weight loss, viral load was higher, and lung inflammation was higher.
Protective effect in mice
The researchers found that these mAbs protected mice by activating the human ACE2 receptor when administered before or after viral challenge. Mice with treatment did not show weight loss, reduced viral load, and decreased cytokine levels, similar to the anti-RBD antibody effect.
What is the impact?
Sections of the NTD are identified by neutralizing and protecting antibodies against SARS-CoV-2 and may act as part of antibody cocktails to induce a selection of escape antibodies or against reducing natural changes in RBD as they occur. ”
The researchers recommend using a combination of antibodies that target the RBD spike and the NTD to increase the level of protection, reducing the risk of mutations escaping, despite being so strong. and the neutralization that the anti-NTD antibodies offer compared to the neutralization of the most powerful anti-RBD antibodies. Vaccines can offer the most stable immunity if developed against multiple antigens, rather than just the RBD spike.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.