Researchers at the University of North Carolina at Chapel Hill have conducted a study examining how the spatial pattern of some genomic RNA segments in coronavirus 2 respiratory distress syndrome 2 (SARS-CoV-2) induces tension, packing , and cycling of the viral genome.
The novel SARS-CoV-2 virus is the agent responsible for the 2019 pandemic of coronavirus infection (COVID-19) which continues to wipe out the world and poses a unique threat to human health. global and global economy.
A major challenge for viruses such as SARS-CoV-2 is the specific and efficient packaging of a large genome into a relatively small capsid while excluding subgenomic viral fragments and cellular nucleic acids.
It is not yet clear how these viruses select the genome from the sub-genomic RNAs that the virus and host transcription generate and regulate to compress into a virion.
Now, using a coarse-grained polymer model, Amy Gladfelter and colleagues have shown that a process called phase separation induces conditions that favor single-packaged. However, the alignment of sequence-stimulated sequencing levels at the end of the genome is critical for genome compression. The elements must be located at each end of the genome for cycling to occur.
The teams say the models presented here could be related to viruses and other cellular systems that contain long nucleotide chains and proteins.
A pre-printed version of the paper is available on the bioRxiv * server, while the article is subject to peer review.
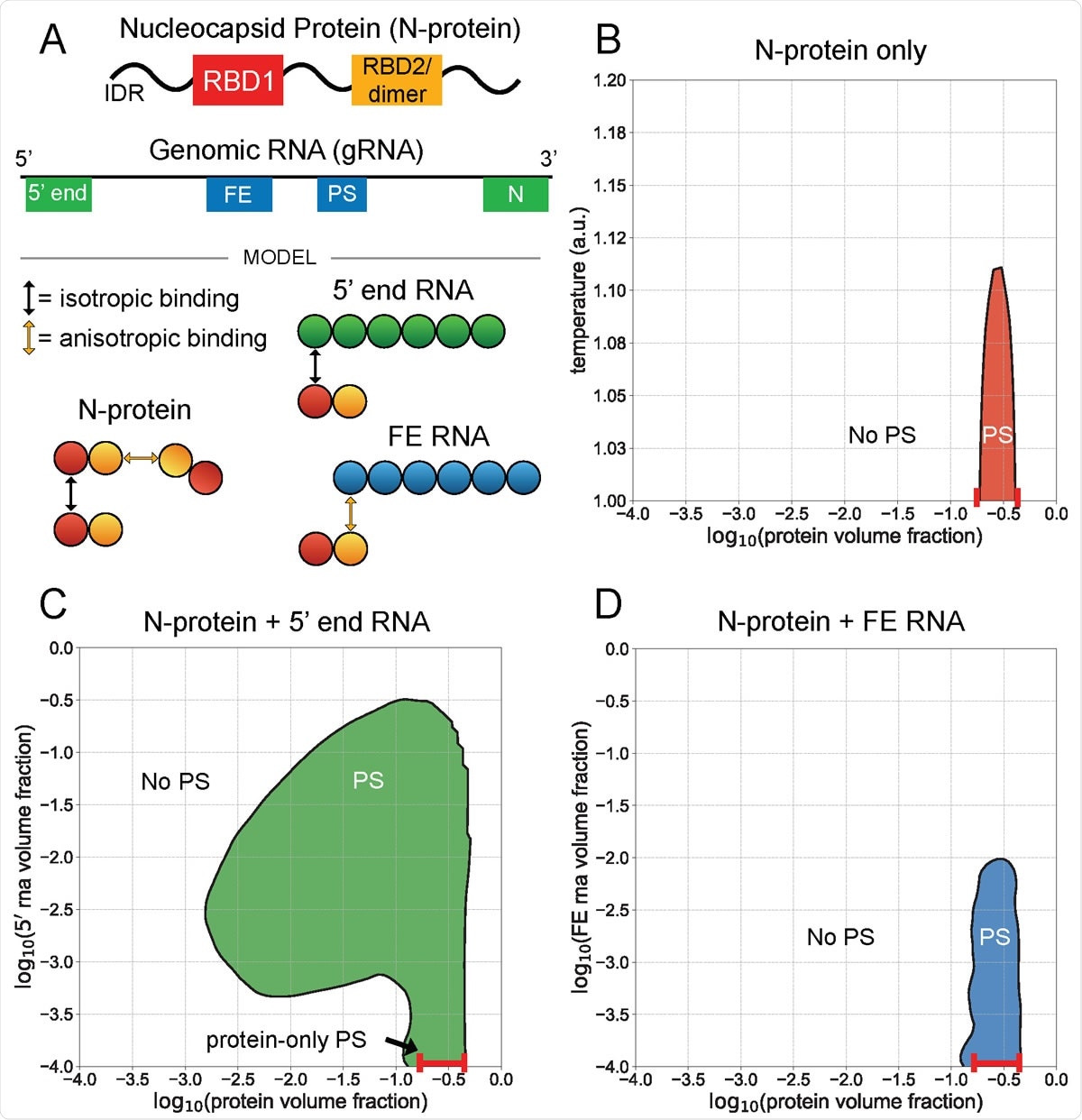
Level end 5 ¢ and FE RNA with N-protein are against phase behavior. A) N-protein is represented as a two-bead series, with the first bead participating in isotropic homotypic interactions, and the second bead participating in anisotropic homotypic interactions. Both 5 ¢ end and FE RNA are about three times larger than N-protein and are represented by six beads each. N-protein interacts with the 5 ¢ end RNA beads through an isotropic bond with its first bead, and interacts with the FE RNA beads through anisotropic bond with its second bead. This interaction with FE competes with N-protein reduction. B) N-protein separates (PS) in a narrow density and temperature range by itself. C) N-protein with 5 ¢ RNA end at 1 au temperature divides over a wider density range than by itself. D) N proteins with FE RNA at temperature 1 au are dissolved at sufficiently high FE RNA concentrations.
Understanding coronavirus reproduction
The COVID-19 pandemic has prompted major research efforts to understand the mechanisms underlying coronavirus reproduction.
Viruses need to selectively package their large genomes in a relatively small capsule while excluding host nuclear acids and viral sub-genomic fragments.
Recent studies have shown that viral proteins required for genome packing can undergo a process called liquid-phase separation (LLPS), where one or two proteins bind viral nucleic acid compatible with the genome.
For SARS-CoV-2, the genomic RNA can condense with the nucleocapsid (N) protein, a protein essential for genome packing in many viruses.
Importantly, some regions of the genomic RNA drive the condensation of the N protein, while others dissolve it.
Previous work by Gladfelter and colleagues found that the N protein passes through LLPS in a manner specific to the order of RNA within different regions of the genome.
“Remarkably, RNAs of the same length can induce or limit phase separation according to the sequences,” the researchers say.
They found that the LLPS progression sequences are in the 5 ¢ and 3 ¢ heads of the genome. This led the team to speculate that phase separation may be relevant to packaging because these sequences occur specifically on the entire genome and would not co-occur on sub-genomic or host nuclear acids.
What did the current study cover?
The researchers used a coarse-grained polymer model to investigate how the spatial pattern of LLPS segments that induce LLPS or inhibit LLPS in the genome could promote specific packing of a single genome.
First, the team analyzed strains of the SARS-CoV-2 genome that exhibits a level of resistance once mixed with the N protein.
The researchers showed that while the 5 ¢ and 3 ¢ margins of the genome promote phase differentiation, the framework element (FE) and central segments release N proteins.
Next, the team examined the spatial pattern of these challenging elements within a full genome model and the impact of this pattern on phase separation and single genome packing, strain and cycle.
The model showed that the best solution for genome packing and compression is to detect elements that stimulate LLPS to 5 ¢ and 3 ¢ ends of the genome and that this displacement is essential for cycling.
Future applications of the model
The researchers say the model can inform future studies investigating the spatial pattern of genomic traits that are experimentally impossible due to the large size of the genome.
“We assume that this model should be applied universally to many viruses as well as cell organelles,” they write.
“Components from several viruses have been shown to undergo phase separation, raising the possibility that a specific spatial pattern of RNA or DNA sequences induced by LLPS may have evolved to optimize genome packing. stimulation in viruses other than SARS-CoV-2, ”concludes the team.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.