The main purpose of any vaccine is to stimulate immune activity and provide long – term protection. A paper was recently uploaded to the preprint server bioRxiv* by Tong et al. (17th February 2021) describes a modern vaccine using adjuvant and recurrent spike proteins against COVID-19 infection that promise the generation of strong neutralizing antibodies, which may support immune responses for years.
What are recurrent vaccines?
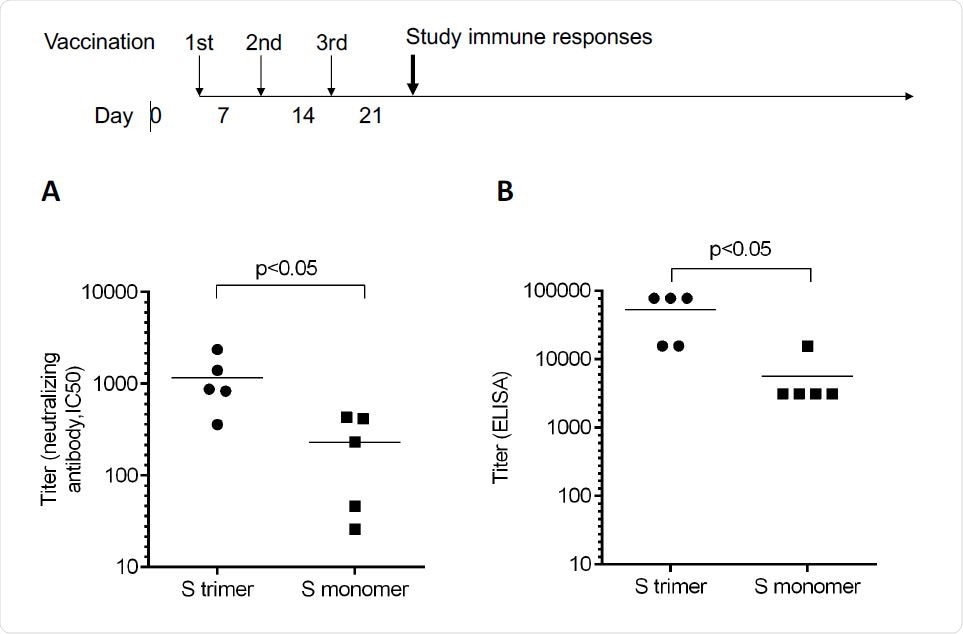
Regenerative proteins are formed in genetically engineered cells, modified to express proteins of interest. In this case, the group selected the full-length spike protein of true respiratory coronavirus 2 (SARS-CoV-2) syndrome, as there are many regions capable of stimulating virus-specific T cells. The spike protein was modified by two protein mutations to keep it in its pre-fusion form, a method similar to the one undertaken by several other groups developing vaccines. PIKA was introduced into the final vaccine, and mice were administered weekly over three weeks with 5 μg of protein and 50 μg of PIKA. The group found that antibodies specific to the spike protein were detected at a titer of more than 50,000 at a height of 21 days, with an average of 1,000, with a higher resolution than achieved by the three approved vaccines (Moderna, Oxford-AstraZeneca , and Pfizer -BioNTech).
Polyinosinic: Polycytidylic acid (poly I: C) is a synthetically synthesized dsRNA sequence that interacts with charge-like receptors on the surface of immune cells to act as an antiviral agent. It is commonly referred to as PIKA when used as a supplemental vaccine in a stable form and has been shown to provide quantitative and qualitative improvements in immune responses when co-administered with an unsupported vaccine.
What are the benefits of recombinant vaccines?
A protein / peptide sequence was performed to test the binding mechanism, and showed that the manufactured antibodies that bound to the engineered spike protein did, in essence, the same as the natural spike protein. -part around the receptor binding area. This shows that the regenerative protein is able to stimulate the immune system similar to the real virus, providing effective and lasting immunity.
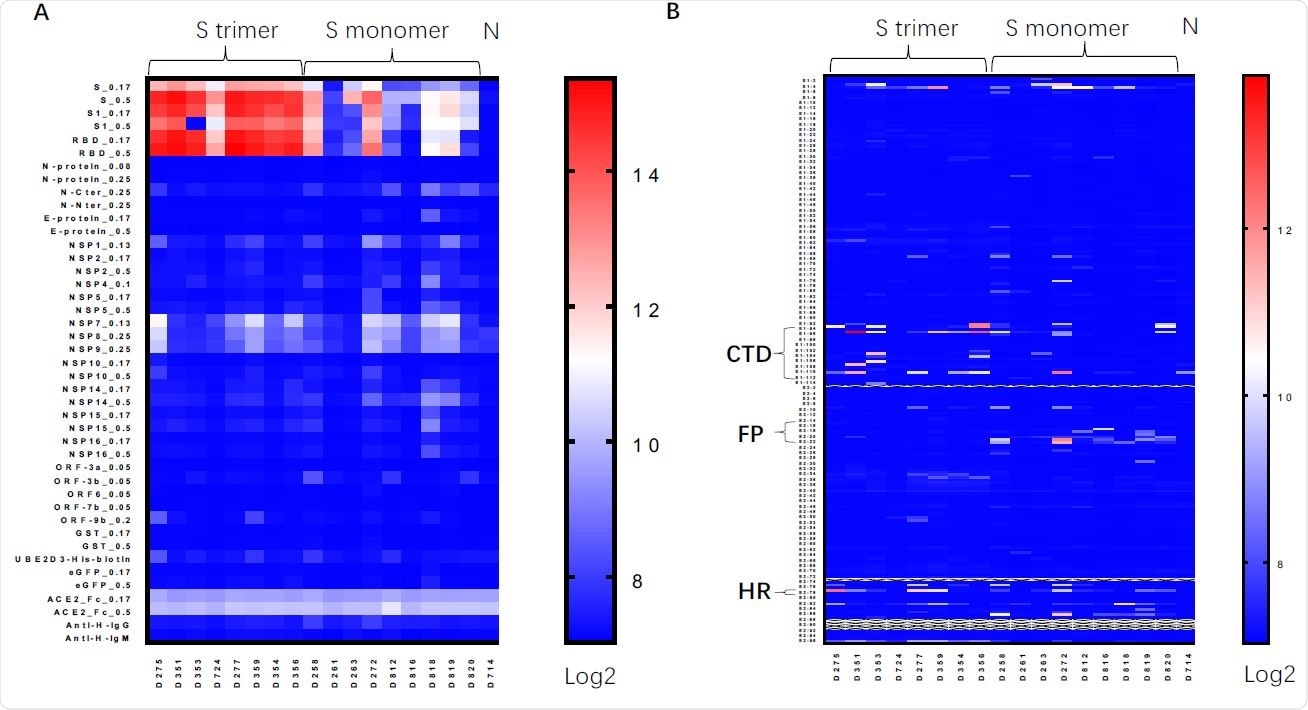
Protein / peptide range of serum antibodies induced by Spike protein vaccines. A. Protein array assay for serum from mice vaccinated with Spike trimer, Spike monomer, nonvaccinated mice, using Spike (S_0.17 and S_0.5 means proteins were printed at 0.17 or 0.5 mg / ml); S1 subunit of Spike, RBD and other viral proteins. B. Linear peptide field using serial peptides of Spike proteins. CTD, C-terminal domain immediately after RBD (Peptides S1-93-S1-113); FP, peptide fusion (Peptides S2-14-S2-23); HR, heptad segments (Peptides S2-78).
The group used Chinese hamster ovary cells (CHO) to express the recombinant protein. CHO cells are popularly used in the production of recycled proteins because they are easily cultured on a large scale and produce valuable amounts of the protein you want, up to 10 grams. every liter of culture. Many decades of working with these cells have allowed precise genetic engineering to produce human biocompatible molecules.
Both adjuvant protein and mRNA vaccines require adjuvant use, such as PIKA, in this case, to stimulate a strong immune response and provide long-term immunity. However, recurrent protein vaccines do not need to be stored at ⁰80⁰C, as with the two most popular COVID-19 mRNA vaccines by Moderna and Pfizer-BioNTech. Other recombinant protein-based vaccines have seen success with longer-lasting immunity than that provided by mRNA vaccines, including hepatitis B and HPV vaccines. In contrast, manufacturing is continuous, more easily scalable, and cheaper.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.