As coronary virus pandemic 2019 (COVID-19) passes the hard milestone of more than one hundred million reported cases, the release of some vaccines offers a unique glimmer of hope that gain some control within a year. Now, a new prospect research paper describes an adenovirus-vectored chimpanzee vaccine that, when introduced intranasally into rhesus macaques, produced a strong immune response and showed protection against infection with the virus that causes COVID-19, that is, chronic respiratory coronavirus syndrome. 2 (SARS-CoV-2). The introduction is published on the bioRxiv* server.
Earlier results
In a previous study, the candidate of the vaccine demonstrated protection against upper and lower respiratory tract infections in mice expressing the viral receptor, the human angiotensin converting enzyme 2 (ACE2). The current study extended these findings to show its effectiveness in nonhuman primates.
An antigen spike SARS-CoV-2
The virus enters the host cell through engagement with a spike antigen with the cell surface receptor ACE2 receptor. The spike protein (S) is therefore an acute immunization target and a focus of the development of therapeutic antibodies. The S protein is purified in several steps, first at the S1 / S2 interface. Subsoil S1 relies on receptor binding at the receptor binding domain (RBD), and then within the S2 domain to produce S2 proteins that mediate viral cell-membrane fusion.
Neutral antibodies recognize the S prefusion protein, which has the RBD in its ‘up’ concentration. The vaccine candidates being developed include DNA / RNA vaccines, nanoparticle lipid mRNA (LNP), inactivated vaccine mRNA, protein-rich vaccines, and vectored viral vaccines.
Prevents viral transmission
Most currently available vaccines, and those undergoing phase III testing, require two doses and are administered intramuscularly. Not only do these conditions require a longer period of time to install immune defenses but they do not provide local or mucosal immunity, allowing viral peeling to continue.
Thus, those who have been vaccinated can still pass on the infectious virus to others even though they are protected from the disease itself. (The Moderna vaccine has been shown to reduce viral peeling of the nose in experimental animals, but there is a lack of human data.)
Tests with intramuscular (IM) vaccines in non-human primates (NHP) show that, despite the protection they offer against COVID-19 pneumonia, the same cannot be said about infection. high airway and, therefore, against viral transmission.
Different vaccine antigens
The researchers described the use of chimpanzee Adenovirus (simian Ad-36) based on the SARS-CoV-2 (ChAd-SARS-CoV-2-S) vaccine that expresses the S protein. when ingested in mice, a single dose of antibodies induced immunity and mediated immunity against the spike protein and prevented infection of both the upper and lower airways.
The spike protein used in this vaccine candidate (ChAd-SARS-CoV-2-S) differs from the other Ad-23 chimpanzee-based vaccine, ChAdOx1 nCoV-19, in phase III trials, by getting rid of a spine that increases spike expression and stabilizes proline mutations to keep the protein in its pre-release form.
The current study used a single intranasal dose of the vaccine in rhesus macaques (Macaca mulatta), followed by SARS-CoV-2 challenge by both intranasal and intrabronchial pathways.
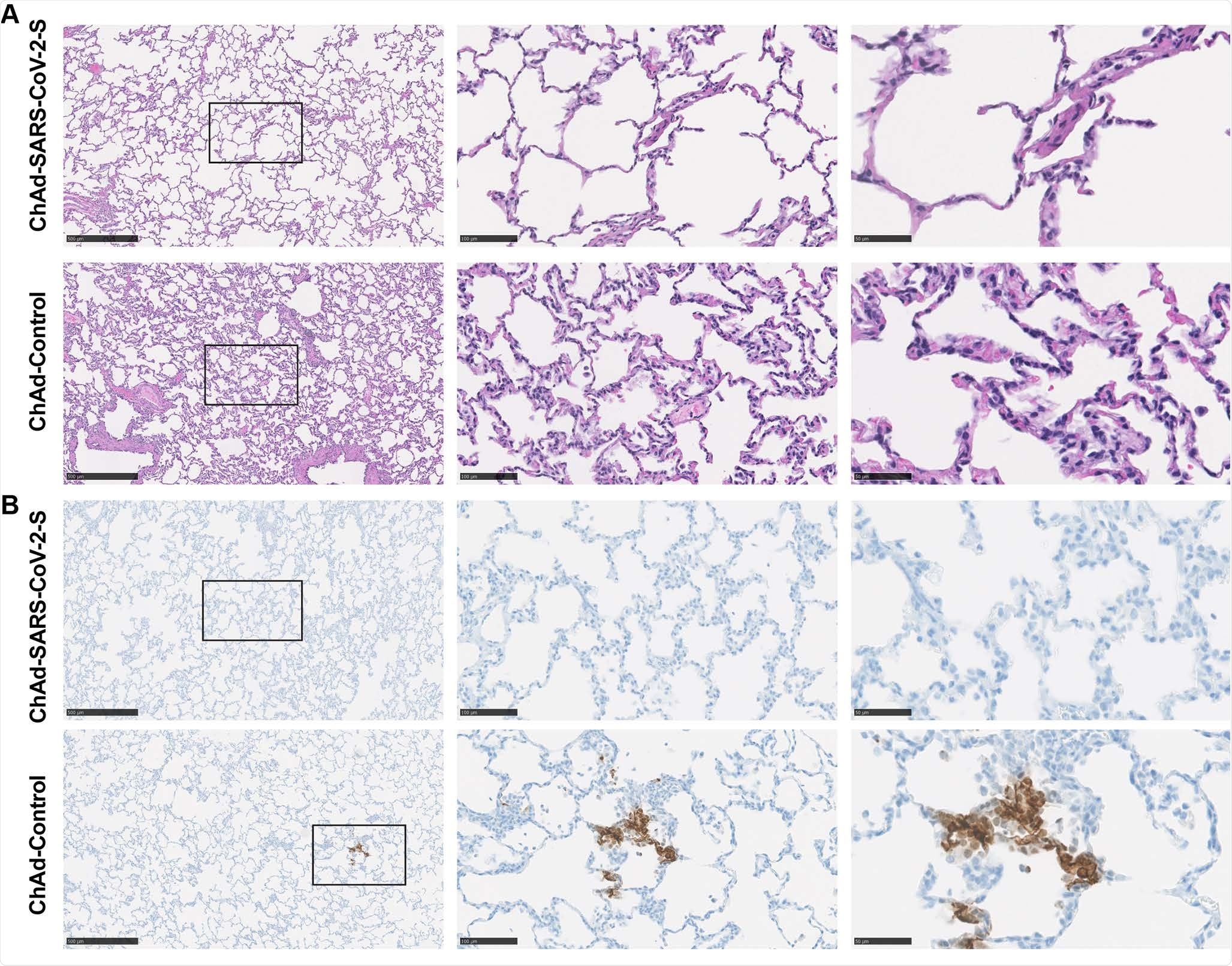
Pathological analysis of vaccinated lung RMs. RMn were vaccinated with ChAd and ChAd-SARS-CoV-2-S control and were challenged. Lungs were cut at 7 dpi. A. Extracts were stained with hematoxylin and eosin and imaged. Each image represents a group of 6 RM. B. SARS-CoV-2 antigen was detected in lung extracts from RMs for conditions described in (A). Images show low- (left; scale, 500 μm), medium- (middle; scale bars, 100 μm), and high power magnification (right; scale bars, 50 μm). Representative images from n = 6 RM per group.
Immunogenic vaccine
They are anti-S, anti-RBD, and neutralizing antibodies. T cell responses were also present.
Protection from local diseases
In nasal swabs, viral RNA loads were reduced in vaccinated animals, and only one had a recognizable infectious virus on the first day after the challenge, compared with four out of six controls. At later times, an infectious virus was not isolated from the nasal swab.
This suggests local infection prevention, with lower viral RNA levels and faster viral clearance.
Protection against lung disease
The researchers also measured the level of infectivity in bronchoalveolar lava flow (BALF) on day 1 and 3 post-challenge and lung (day 7), from exposed animals. One in six samples on day 1 was positive for an infectious virus, vs. each control sample. The viral titer was also smaller by three orders of magnitude.
On day 3, only one sample from a control animal was positive for an infectious virus. Viral RNA was increased one-hundred-fold and fifty-fold, on days 1 and 3, respectively, in control of BALF samples compared with vaccinated animals, supporting viral clearance.
The lung figs in controls also showed a much higher level of viral RNA in vaccinated vs. control animals on day 7. In fact, the higher the neutralizing antibody titer, the lower the viral RNA in controls. BALF. This correlation was more sensitive than the anti-S IgG, indicating that the titer of antibody-neutralizing may be dependent on the level of protection this vaccine offers.
Viral antigen staining in the lungs of vaccinated animals was not present, against four of six control animals.
What is the impact?
Both at the site of ingestion and in distant cigarettes, a single dose of the ChAd-SARS-CoV-2-S vaccine could significantly reduce the incidence of infection and viral transmission. Indeed, there was very little malignant lung pathology, or disease, in both control and vaccinated animals, despite the use of high doses of disease and the introduction of the infectious virus by both intranasal and intrabronchial pathways. This limits any determination of the efficacy of the protection of this vaccine against SARS-CoV-2-induced infection.
“Because this single-dose intranasal vaccine protects against SARS-CoV-2 in non-human primates, it is a promising candidate for limiting SARS-CoV-2 infection and transmission in humans.. “Further research could focus on ways to increase the level of protection, including the possible use of a homologous or heterologous elevated dose with the same vectored adenovirus vaccine.
It is another encouraging sign that disease progression does not depend on vaccination. However, further research is needed to compare the immunity achieved by intranasal and IM administration of this vaccine, as well as the time frame of immunity over time after intranasal vaccination with ChAd-SARS-CoV- 2-S.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.