A recent study from France showed that the UK variant of coronavirus respiratory syndrome 2 (SARS-CoV-2) – also known as B.1.1.7 variant – can reproduce much more effectively in bronchial epithelium reconstructed human beings, which may explain why it is spreading so rapidly in the human population. The study is currently freely available on the bioRxiv * preprint server.
Since its inception in 2019, circulating populations of SARS-CoV-2 have been known to continuously acquire genetic diversity. As a result, within a few months, D614G spike mutations were dangerous in all viral populations, however, in the absence of evidence for higher 2019 coronavirus infection or mortality (COVID-19).
In September 2020, a nominal 20I / 501Y.V1 variant from the B.1.1.7 line appeared in the United Kingdom, with subsequent rapid deployment around western Europe and the United States. Many lines of evidence show that this so-called ‘UK variant’ can spread much faster and more efficiently compared to previous European variants – especially in younger people.
Moreover, new studies even show a potentially greater death toll as a result of UK stress, which, along with increased transmission, can trigger waves of disease. Nevertheless, there is still a lack of adequate biological evidence of different phenotypic traits compared to original European variants.
In this study, a research group from the University of Aix-Marseille in France (led by Dr. Franck Touret) decided to evaluate the reproducibility of the UK variable in different cell models, using European snoring. D614G ancestral (line B1) for comparison purposes.
Highly specific / rational cell models
In this study, the reproducibility of the viral variant 20I / 501Y.V1 (strain UVE / SARS-CoV-2/2021 / FR / 7b was separated in February 2021 in Marseille, France) in vitro and ex vivo, using the B.1 BavPat D614G line circulated in Europe in February / March 2020 for comparison purposes.
The first experiments were performed in two commonly used cell lines for SARS Cov-2 culture: VeroE6 / TMPRSS2 (African green monkey kidney cells) and Caco-2 (i.e., human colorectal adenocarcinoma cells).
After revealing a very similar replica kinesiology, the researchers assessed the replica fitness of both strands using a model of reconstructed human airway epithelial cells of bronchial origin.
Notably, after ingesting the epithelia through their apical side at a disease multiplication of 0.1 (made to mimic the natural pathogenesis of infection), the researchers looked at the inhibition of new viruses. at the apical side 2-4 days after infection and measured the intracellular yield of viral genetic material at day 4.
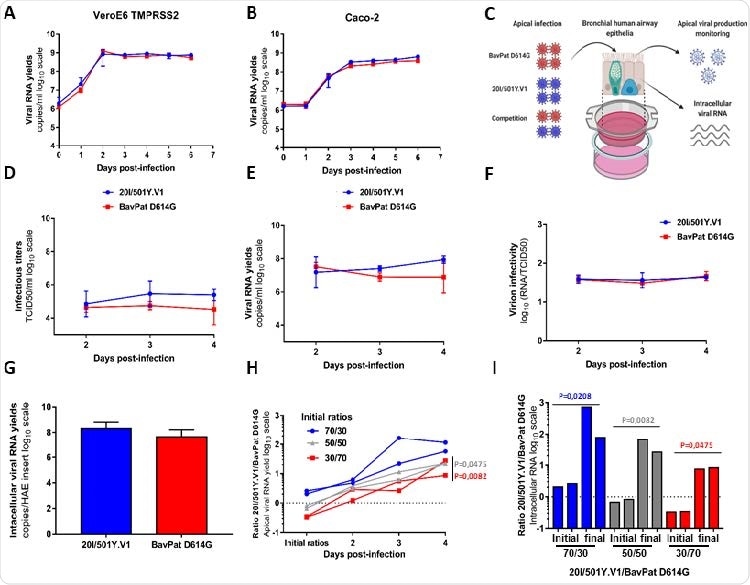
In vitro and ex vivo reproducibility of 20I / 501Y.V1 variant compared to B.1 D614G sequence. (AB) Reproductive kinetics in VeroE6 TMPRSS2 (A) and Caco-2 (B) cells. Viral replication was assessed using the RT-qPCR assay. (C) Graphical representation of experiments with reconstructed human airway epithelium (HAE) of bronchial origin. (DE) Kinetics of virus inhibition at the apical side of the epithelium measured using assay TCID50 149 (D) and assay RT-qPCR (E). (F) Measurement of virion infections (i.e., the ratio of the number of infectious grains over the number of viral RNA). (G) Intracellular viral RNA yield measured at 4 dpi using RT-qPCR assay. (AG) Data represent the mean ± SD of triple. No statistical difference was observed between the two viral sequences (p> 0.05; unprepared Mann-Whitney test). (H) Follow the ratios 20I / 501Y.V1 / BavPat D614G at the apical side. Each line represents results from HAE inputs. (I) Individual 20I / 501Y.V1 / BavPat D614G ratios estimated from intracellular viral RNAs at 4dpi (I). P-values (HI) were determined according to the initial ratios using the Kruskal-Wallis test and then the Dunn uncorrected post-hoc study. The graphical representation was created by BioRENDER.
A new strain over the old one
The study has shown that infectious titers and yield of viral genetic material at the apical side of the cells were slightly higher for the UK variant at days 3 and 4 of the disease. The same was valid for intracellular production of viral genetic material on day 4 of the disease.
Nevertheless, these differences were not significant, although relative virion infectivity (measured as the ratio of the number of infectious grains over the number of viral RNA) was estimated. or genetic material) for both viral sequences at each sampling time.
The highlight of this study was when the two viruses competed in human reconstructed bronchial epithelium because the UK variant subsequently ejected the sex of their ancestors – although whatever the original ratio was used in this study.
Understanding strain replacement
“Our results showed that the 20I / 501Y.V1 is more suitable than the BavPat D614G in reconstructed bronchial human epithelium”, say study authors here bioRxiv paper. “This can be explained by the presence of N501Y mutation in the receptor binding domain (RBD) of the spike protein, which promotes viral binding to the ACE2 receptor”, they add.
In any case, this could give the virus specific fitness benefits, as recently demonstrated in research projects using engineered viral rays. In particular, similar results were observed with the D614G variant, where the new G614 series surpassed the original D614G series when put into competition.
All that said, this study could contribute to our better understanding of the replacement of SARS-CoV-2 20I / 501Y.V1 (or UK) strains. The emergence of various changes raises several questions regarding the future course of this pandemic. Therefore there is a great need for more research efforts in this area.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.