As researchers and physicians strive to find effective and safe therapeutic treatment to combat coronavirus disease 2019 (COVID 19), an interesting new study published in the bioRxiv* a preprint server suggests that inhibition of cholesterol transport through its high B-1 cell receptor scavenger receptor (SR-B1) may inhibit viral entry by up to 80%. This highlights a potentially therapeutic pathway for further development, not only for COVID-19 but other infections caused by viruses with the same basic mechanism.
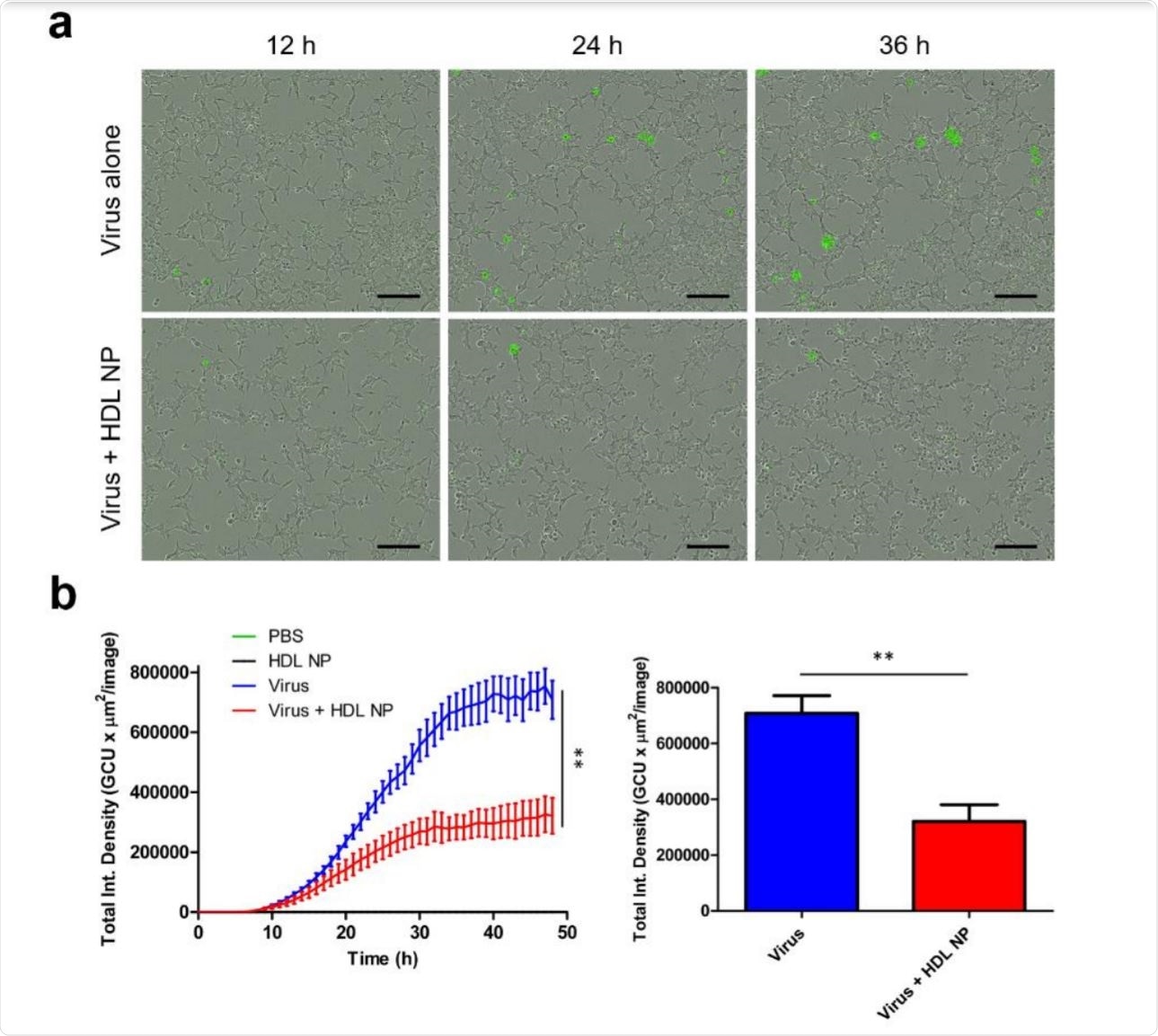
Treatment of HDL NP inhibits pseudovirus SARS-CoV-2 infection in HEK293 (ACE2) cells. Image credit: https://www.biorxiv.org/content/10.1101/2020.12.14.420133v1.full.pdf
SARS-CoV-2 entry and risk factors
The acute respiratory coronavirus-2 (SARS-CoV-2) acute respiratory syndrome is a single-stranded RNA virus that causes COVID-19. Like other enveloped viruses, infection occurs in two stages, first the host host receptor binding and then membrane fusion leading to virus entry into the host cell.
Like other coronaviruses, this virus contains three structural proteins in its capsid, membrane (M), envelope (E), and spike proteins (S). The spike protein is dependent on viral entry.
Many risk factors have been identified for severe or acute COVID-19, including obesity, advanced age, and diabetes mellitus. The median age of individuals who die of COVID-19-related causes is 78 years. The reason for this accumulation of risk factors with unbalanced mortality is now emerging.
Earlier, several studies showed that the virus (SARS-CoV), which was responsible for the earlier SARS pandemic, showed a clear correlation between its infectivity and cholesterol in the host cell. . This is also the case with the common virus, SARS-CoV-2, according to a recent study.
The virus binds to the host cell receptor, the enzyme 2 that converts angiotensin (ACE2), to enter. However, this process has recently been shown to be dependent on receptors, known as SR-B1).
SR-B1 and HDL duty
This SR-B1 receptor binds high-density native lipoproteins (HDL), which are rich in cholesterol – usually nicknamed ‘good cholesterol’ because they remove cholesterol from the body’s cells to transport it to the liver, for extraction, and to various livers where it is used to synthesize steroid hormones. This is called reverse transport of cholesterol.
HDLs are nanoparticles with a range of physiological functions. They have anti-inflammatory properties and maintain both epithelial and endothelial inhibition activity. They also participate in the stem transport of cholesterol, allowing them to alter cholesterol metabolism within cells.
SR-B1 can interact with HDL to allow the virus to enter airway cells. This receptor is present on ae cells, immune cells, and type II alveolar cells, thus agreeing with the targeting of SARS-CoV-2 on the lungs. This receptor has previously been detected as a co-receptor for other viruses, including hepatitis C virus and plasmodia.
SARS-CoV-2 has been shown to achieve host cell entry at cholesterol-rich membrane areas. Cholesterol also helps the cell move the host cell receptor, angiotensin-converting enzyme 2 (ACE2) to these areas while strengthening the cell’s affinity for SARS-CoV-2.
Based on these findings, the researchers suggest that the virus can be explained as a cause of serious disease or death in high-risk groups and that cholesterol lowering may be a viable approach. therapeutic out of debt.
Most research on COVID-19 therapies focuses on inhibiting virus-ACE2 interactions, using specific antibodies or decoy molecules to pull the virus away from the host cell target. In this experiment, the researchers tried to use knowledge of the role of cholesterol in viral entry to alter the cell membrane, thus making it hostile to viral entry.
Both SARS-CoV and SARS-CoV-2 are known to use lipids rich in cholesterol and in another type of lipid called monosialotetrahexosylganglioside 1 (GM1), to enter mammalian cells in culture. When treated with a fertilizer called methyl-β-cyclodextrin (MβCD) that removes cholesterol, before the cells became exposed to these viruses, the rate of infection was greatly reduced.
Recent studies have shown that cholesterol synthesis in the body may be necessary for access to the host cells. The study examines the extent to which nanoparticle formation, through targeted cholesterol reduction, can inhibit SARS-CoV-2 ingestion.
HDL nanoparticles show great promise
The scientists had developed synthetic HDLs for their research purposes. In the current experiment, they used an inorganic basic nanoparticle (NP) around which HDL-related proteins (apolipoprotein AI) and lipids were accumulated. This surface appearance on HDLn caused these NPs to bind tightly to SR-B1.
However, their downstream effects on cholesterol are different members, due to the lack of cholesterol esters at their base. As a result of the reduction in cholesterol within the cell after binding these NPs, the cell synthesizes more cholesterol to make it up.
When examining their potential for therapeutic use, these NPs demonstrated their ability to powerfully inhibit the entry of exosomes, which are extracellular lipid bones. These are very similar to viruses in some ways. Second, the ability to alter cell cholesterol has led to the use of these HDL NPs as antitumor molecules to treat cancers that require SR-B1-dependent cholesterol uptake.
Third, by altering the type of lipids accumulated on the surface of these NPs, they can be made to target Gram-negative bacterial lipopolysaccharide (LPS) and inhibit LPS-mediated strong pro-inflammatory cytokines such as NF-κB.
Blocking cholesterol-based viral entry
Incorporating these proven uses, the researchers used these granules to reduce cholesterol in cell membranes and studied the effect of such a reduction on SARS-CoV-2 pseudovirus entry.
They found that when cells were treated with HDL NPs at a dose of 50 nM simultaneously with exposure to the pseudovirus, there was a significant reduction in infectivity by 5555% and 80%, according to the line cell used. This difference in inhibition is directly related to the SR-B1 sensitivity level.
When the expression of this receptor was blocked, there was a corresponding decrease in infectivity, supporting the postulate that SR-B1 is a receptor for the virus. And finally, treating such cells with HDL NPs as well as reduced viral entry, but not as much as handling HDL NP alone.
Decision and impact
Viral entry of SARS-CoV-2 is reduced when SR-B1 is depleted, suggesting that SR-B1 is a co-receptor for SARS-CoV-2; moreover, data support that HDL NPs target SR-B1 to prevent SARS-CoV-2 entry. ”
Therefore, HDL NPs deserve to be studied for their ability to prevent infection with SARS-CoV-2. They could also be developed as a potential treatment for all viral infections that are dependent on the presence of cholesterol in the cell membrane, and which require lipid rafts, for successful admission. in host cells.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.