A meeting with doctors from Wuhan gave a strong insight to Professor Laura Manuelidis, MD, from Yale School of Medicine in early 2020. A special feature in one slide that prompted the doctors gave an interesting study: the pathology of interstitial pneumonia was highly controlled. regional with many large cells that were more consistent with myeloid line.
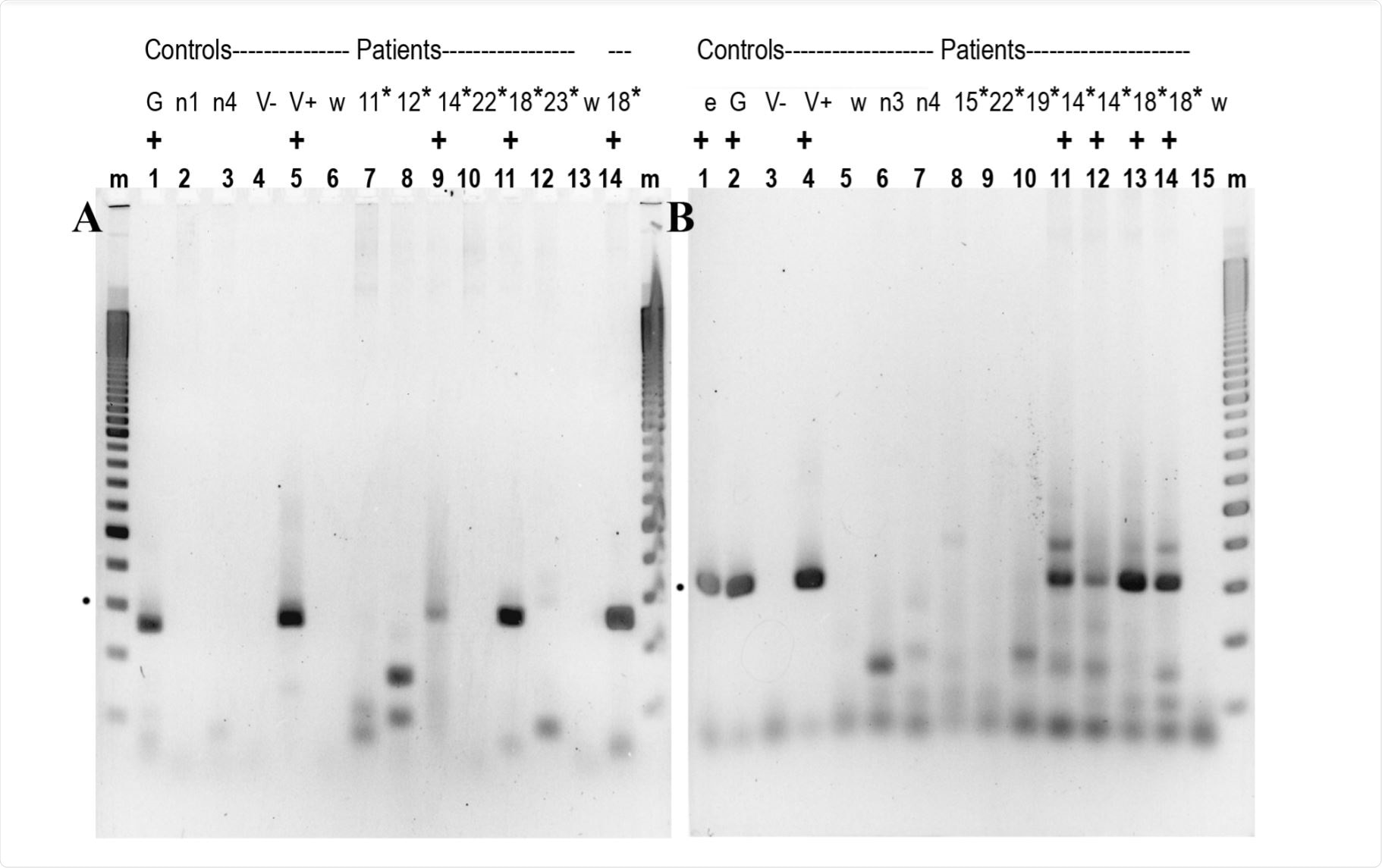
2.5% agarose gel showing RT / qPCR results. Image credit: https://www.biorxiv.org/content/10.1101/2020.12.16.423113v1.full.pdf
Her team decided to find out if the severe respiratory disease coronavirus syndrome 2 (SARS-COV-2) of Peripheral Blood Mononuclear Cells (PBMC) is occurring and may be the source of the multiple transmission. organs in the human body. Viruses are known to release free viruses into the bloodstream or travel inside the white blood cells in the host. Many viruses affect PBMC which can be a reservoir for stability.
SARS-CoV-2 migrating myeloid cells may be able to reside and survive infectious and malignant pathology in the brain and other organs. The COVID-19 pandemic caused by the SARS-CoV-2 has exhibited more (expected) brain and heart pathologies.
COVID-19 postoperative disease, neurologic disorders, and neuropathology, e.g., thromboemboli, infarction, radiologic changes consistent with autoimmune encephalitis, and even the presence of SARS-CoV-2 in neurons are reported. Little is known about the immediate or late effects of viral or late-onset SARS-CoV-2 on the brain.
Professor Laura Manuelidis and her team from Yale medical school recently discovered evidence of SARS-CoV-2 nucleocapsid in peripheral blood mononuclear cells (PBMC) bioRxiv* introduction publication. They found more than 300nt of RARS nucleocapsid SARS-CoV-2 in PBMC – showing that these cells can act as channels for the spread of the virus, or viral elements, to other organs.
The researchers first analyzed a 72bp short-acting nucleocapsid (NC) sequence of the SARS-CoV-2. The NC proteins protect the viral genome. Therefore, they further analyzed longer (301nt) domains next to NC with RNA / qPCR.
The study group included 7 male and 7 female patients aged 24–82 years and 4 randomized unprotected randomized volunteers. PBMCs from these 14 patients were administered 2-6 days after admission to the hospital with a COVID-19 positive swab test.
In this study, the patients were sampled only once in the disease. Because of this, only 20% of patients were positive.
In 2 PBMC patients (but not in the unprotected controls), they found longer NC sequences. These were positive as early as 2–6 days after admission to the hospital. They confirmed that NC was further present by placing the order.
Coronaviruses are complex and can elicit autoimmune responses that damage the brain and other organs. The respiratory coronaviruses, prior to the SARS-CoV-2 strain that causes human colds, are known to be neuroinvasive. Its viral RNA is found in the parenchyma of the brain as well as in myeloid microglia in culture.
Sudden Deaths Occurring Often from non-hepatic “viral” diseases on routine autopsy showing classic acute and myeloid lymphocytic infiltrates in a completely normal heart. Some of these may be due to coronavirus strain. The recent fall of healthy young athletes could be caused by the release of SARS-CoV-2 PBMC leading to vascular microthrombi in the heart and brain.
In addition to the neural olfactory distribution, the researchers suspected the role of a subset of blood monocytes in the transport of SARS-CoV-2 into the brain with subsequent development of neuropsychiatric symptoms.
The researcher advised on the method used – more quantitative NC RT / qPCR Cts should start with> 5e7 PBMC per person. They explain that this number of PBMCs allows the classification of PBMC subsets that mimic SARS-CoV-2sequences.
The researchers therefore warrant timely assessments at different stages of SARS-CoV-2 exposure; they can be used in a diagnostic way.
The team suspects that a circulating type of myeloid cell appears to carry the viral sequences, especially as these cells reside in cigarettes and act as a latent source for infectious disease. and / or the promotion of harmful immunity. It is important to identify the progressive cell subtypes and also to see if these cells have the infectious virus.
This study is important in suppressing antibody and also other new strategies to prevent progressive transmission to other organs. Research on the next stage in this study will help to identify the advanced cell subtype as well as therapeutic approaches that specifically target these cells to prevent the early or partial release of SARS-CoV-2. , to other organs, the researchers write.
The presence of the RNA nucleocapsid SARS-CoV-2, particularly in the migration of myeloid cells, may be due to some of the pathologic manifestations found in other organs such as the brain, heart, and lungs – as has been watched by this team in the case presented from Wuhan.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical / behavioral practice, or be treated as information established.
Magazine Reference:
- Detection of long-acting nucleocapside sequences of SARS-CoV-2 in peripheral blood monocytes collected shortly after admission to hospital Nathan Pagano, Maudry Laurent-Rolle, Jack Chun-Chieh Hsu, Yale IMPACT research team, Chantal BF Vogels, Nathan D Grubaugh, Laura Manuelidis. bioRxiv 2020.12.16.423113; doi: https://doi.org/10.1101/2020.12.16.423113