With the advent of COVID-19 (coronavirus disease 2019), researchers have been closely examining the complex interaction between acute respiratory coronavirus 2 syndrome (SARS-CoV-2), the human immune system, and severe COVID-19 disease.
Signal proteins called cytokines release up to dangerous levels, leading to a ‘cytokine storm’ that damages the body’s (self) cells. High levels of intravascular neutrophil extracellular traps (NETn) or inflammatory cell residues increase thrombosis. Homogeneously, all of these results lead to macrovascular and microvascular thrombosis during SARS-CoV-2 infection.
Although endothelial cell activation has been identified as part of the thrombo-inflammatory storm COVID-19, the intermediates above this function are unknown. Recently medRxiv* preprint publication, researchers report for the first time that the serum from COVID-19 patients – which contains circulating antiphospholipid antibodies – activates endothelial cells to express surface adhesion molecules ( namely E-selectin, VCAM-1, and ICAM-1). These surface adhesion molecules are famous for their role in thrombosis.
Find the interdisciplinary team led by Dr. Jason S. Knight and Dr. Yogendra Kanthi that this activation could be completely attenuated by reducing the samples of Immunoglobulin G (IgG) for at least a subset of serum samples.
“Prothrombotic aPL Abs strongly associated with these phenotypes suggests a new way by which these autoantibodies can survive thrombo-inflammation in COVID-19.”
Scientists studying COVID-19 increasingly elucidate the role of autoantibodies, which invade the cell surface, activate endothelial cells, platelets, and neutrophils, scavenging the blood-endothelium leading to thrombosis. This team had previously found that COVID-19 IgG fractions were enhanced for antiphospholipid antibodies (aPL Abs) strongly activating neutrophils in vitro while they also enhance thrombosis when introduced into mice.
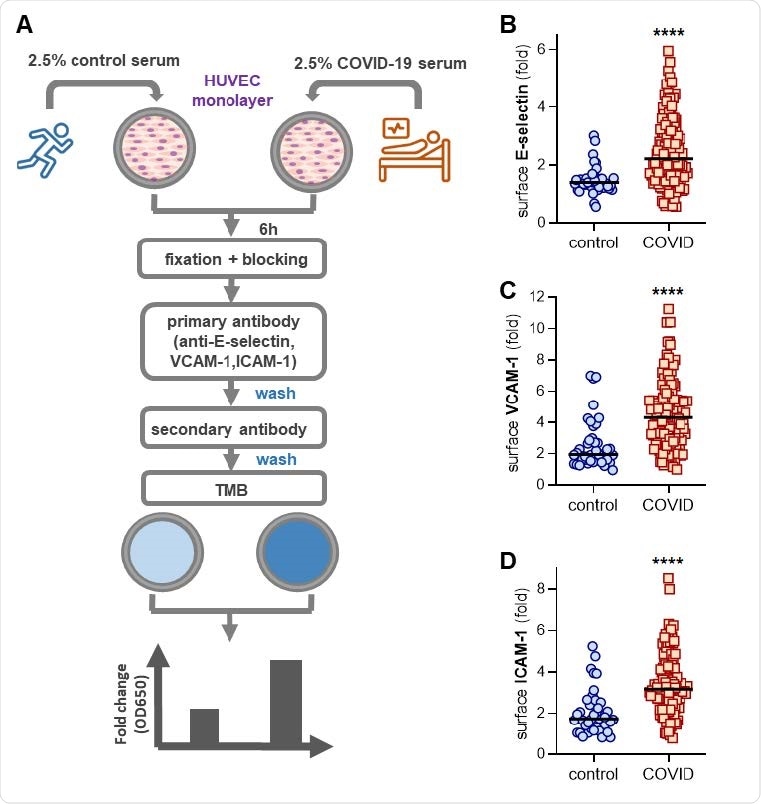
Activation of human umbilical vein endothelial cells (HUVEC) by COVID-19 control or serum. (A) Scheduled workflow for in-cell ELISA. HUVEC was cultured for 6 h with serum from either healthy controls (pre-pandemic collection) or hospitalized patients with COVID-19. The cells were then fixed and a surface volume of E-selectin (B), VCAM-1 (C), or ICAM-1 (D) was measured. Median values are denoted by horizontal lines. Groups were examined by the Mann-Whitney test; **** p <0.0001.
In the present study, the researchers express the hypothesis that circulatory factors such as neutrophil extracellular trap residue (NET), D-dimer, or C-reactive protein could predict the serum samples. COVID-19 (n = 118) becomes the most active culture. endothelial cells.
As expected, they found an association between serum NET residues (cellless DNA, myeloperoxidase-DNA ratios, citrullinated histone H3) and surface upregulation of E-selectin, VCAM-1, and ICAM-1 on endothelial cells. . However, interestingly they also found the presence of circulating antiphospholipid antibodies (specifically anticardiolipin IgG and IgM and anti-phosphatidylserine / prothrombin (anti-PS / PT) IgG and IgM), as markers of serum COVID- 19 with strong endothelial cell activation- ability.
The study group included 118 hospitalized patients with moderate-to-severe COVID-19 at an academic hospital. The serum from these COVID-19 patients was diluted in culture media and then added to human umbilical vein endothelial cells (HUVEC). Any expression of surface action symptoms was confirmed after 6 h with normal ELISA in a cell. For control, the serum samples were collected from 40 healthy individuals.
The researchers found that the COVID-19 samples induced activated endothelial cell phenotype, evidenced by increased surface area of the leukocyte adhesion molecules E-selectin, VCAM-1, and ICAM-1.
Looking for upstream mediators that activate the endothelial cells, the researchers measured serum NETn and common symptoms of thromboembolic inflammation: C-reactive protein, D-dimer, and neutrophil calprotectin. Their results show a moderate correlation between NETn, thrombo-inflammation, and serum COVID-19 ability to activate endothelial cells.
In this study, the researchers found a strong correlation between the four antibodies (IgG and IgM isotypes of two types of aPL Abs (anticardiolipin and anti-PS / PT) and the three markers of endothelial cell activation (E-selectin, VCAM-1, and ICAM-1), reveal their role in the activity of endothelial cells.
In summary, these data indicate that serum COVID-19 contains properties capable of activating the endothelial cells. The researchers cite and discuss the recent reports of autoantibodies in COVID-19 patients and their apparent association with the severity of the disease.
“Taken together, these findings suggest a pathological role for autoantibodies in COVID-19 with mixed effects on immune function, thrombosis, and similar clinical outcomes.”
One of the potential clinical implications of the study suggested by the researchers is to consider whether patients with moderate-to-severe COVID-19 should be screened for aPL Abs to be evaluated. the risk of thrombosis and progression to respiratory failure. Thereafter, such patients may benefit from other treatment strategies.
Importantly, the results from this study also suggest possible alternative therapies, including monoclonal antibody-mediated infusion of plasmablasts, the specific inhibition of reactive B cells against phospholipids and phospholipid-binding proteins, endothelial cell stability therapies, and pathogen-antibody Fc glycosylation or afucosylation are all worthy of future research, the researchers write. This study calls for further studies to understand how APL-related IgG fractions activate the endothelial cells.
* Important message
medRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.
Magazine Reference:
- Cell-activating endothelial antibodies in COVID-19 Hui Shi, Yu Zuo, Alex A. Gandhi, Gautam Sule, Srilakshmi Yalavarthi, Kelsey Gockman, Jacqueline A. Madison, Jintao Wang, Melanie Zuo, Yue Shi, Jason S. Knight, Yogendra Kanthi, medRxiv, 2021.01.18.21250041; doi: https://doi.org/10.1101/2021.01.18.21250041, https://www.medrxiv.org/content/10.1101/2021.01.18.21250041v1