Researchers in the United States report that the recent severe variant of coronavirus respiratory syndrome 2 (SARS-CoV-2) – P.1 – that has recently emerged in Brazil shows a level of anxiety against neutralization the antibodies.
The SARS-CoV-2 virus is the agent responsible for the 2019 coronavirus outbreak (COVID-19) which is a persistent threat to humans and has now infected more than 2.56 million people. across the globe.
The team from Columbia University in New York found that, in addition to resisting several neutral monoclonal antibodies (mAbs), the P.1 variant is also six times greater against neutralization with convalescent plasma and more twice as stable as sera from vaccines than wildtype virus.
However, the loss of neutralization activity shown by convalescent plasma and vaccine sera against P.1 was not as great as the reported loss of activity against the B.1.351 variant identified in South Africa.
David Ho and colleagues say this indicates that the risk of re-infection and reduced vaccine efficacy that is P.1 may not be as severe as the risk of B.1.351.
A pre-printed version of the research paper can be found on the bioRxiv* server, while the article is subject to peer review.
Recent differences appear against neutralization
Several studies have shown that the recently identified SARS-CoV-2 variables B.1.1.7 and B.1351 in the UK and South Africa, respectively. These harbor mutations counteract neutral activity previously induced by disease or immunization.
The P.1 variant that has emerged in Northern Brazil has been shown to induce ten mutations in the viral spike protein – the main structure used by SARS-CoV-2 to bind to and cell infection.
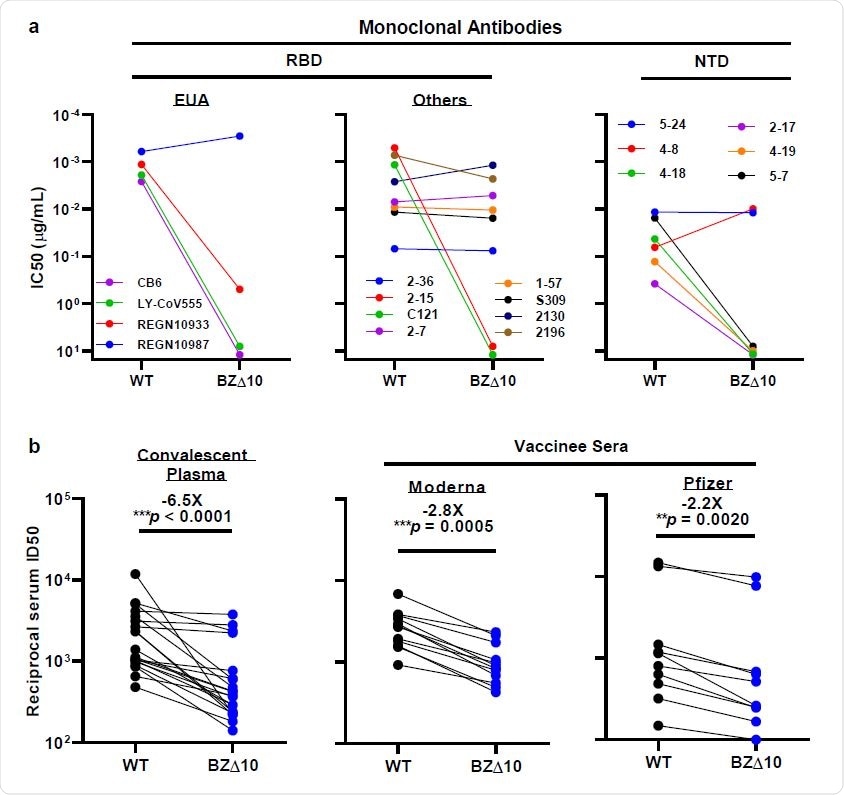
Neutralization of WT and BZ △ 10 pseudoviruses with mAbs, convalescent 178 plasma, and vaccine sera. a, Changes in IC50 neutralization of selected RBD and NTD mAbs. b, Changes in congenital ID50 plasma neutralization of convalescent plasma values and bilateral ID50 serum values for Moderna or Pfizer vaccinated individuals. There is a folded average change in ID50 compared to the written WT above the p values.
In addition to an established D614G mutation that became the dominant domain early in the pandemic, there are three mutations in P.1 (K417T, E484K, and N501Y) in the spike receptor binding domain (RBD), five mutations (L18F, T20N, P26S, D138Y, and R190S) in the N-terminal range (NTD), and one mutation (H655Y) near the furin acting site.
The three RBD mutations are the same as those found in the RBD of B.1.351, a variant that has been shown to resist neutralization with some mAbs, convalescent plasma, and sera from vaccines.
“This new variant could threaten the effectiveness of conventional mAb treatments or vaccines as it divides mutations at the three RBD residues by B.1.351,” wrote Ho and team.
What did the researchers do?
The pseudovirus researchers created a SARS-CoV-2 containing the 10 mutations (BZ∆10) found in the P.1 variant and evaluated the likelihood of neutralization with 18 mAbs. , 20 plasma samples from individuals and 22 serum samples from vaccines.
When the team tested BZ∆10 against four mAbs licensed for emergency use (EUA), including imdevimab, casirivimab, bamlanivimab, and etesevimab, imdevimab was the only one that retained its neutral activity. original. The neutral activity of the remaining three was significantly reduced or underestimated.
“Here we report that P.1 is indeed resisting neutralization with several RBD-led mAbs, including three with EUA,” wrote Ho and colleagues.
mAbs aimed at the RBD and NTD
Next, the team tested eight mAbs targeting RBD, which showed that two previously strong mAbs did not exhibit neutral activity against BZ∆10.
“Overall, these results are similar to those observed for B.1.3513, which should come as no surprise given that the RBD mutations are triple in P.1 and B. 1.351 in much the same way, ”say the researchers.
The pseudovirus BZ∆10 also strongly opposed neutralization with four of six mAbs aimed at NTD. However, two mAbs targeting the antigenic superstructure in NTD that have completely lost neutral activity remained against B.1.3513, still active against BZ∆10.
“The protection profiles are very different for P.1 and B.1.351, reflecting the specific sets of seals in NTD,” the team wrote.
To understand the specific mutations responsible for this nesting pattern, the researchers tested the NTD-targeted mAbs against a panel of pseudoviruses that contained only one of the five NTD mutations. (L18F, T20N, P26S, D138Y, and R190S) found in P .1.
Not surprisingly, the two mAbs that were active against BZ∆10 maintained neutral activity against all single-mutation pseudoviruses. Of the remaining four mAbs, one or more of the five mutations reported a loss of neutral activity against BZ∆10.
What about convalescent plasma and vaccine sera?
When the researchers tested 20 convalescent plasma samples for neutralization activity against BZ∆10, they observed a 6.5-fold decrease in neutral activity against BZ∆10, compared with wildtype pseudoviruses.
Finally, serum samples from 12 who received the Moderna mRNA-1273 vaccine and 10 who received the Pfizer BNT162b2 vaccine were evaluated for neutralization against BZ∆10 and wildtype pseudoviruses.
A decrease in neutralization activity against BZ∆10 was observed for all samples, but the magnitude of the loss was moderate (2.8-fold for Moderna; 2.2 fold for Pfizer), compared with the loss against B. 1.351 pseudovirus (8.6 fold for Moderna; 6.5 fold for Pfizer).
What do the authors suggest?
“Both convalescent plasma and sera vaccine show a significant loss of neutral activity against P.1, but the reduction is not as great as that reported against B.1.351,” the researchers say. “Therefore, the risk of re-infection or lower immune defenses with P.1 may not be as severe as B.1.351.”
The team also states that, since the RBD mutations are essentially the same between the two variables, the difference in their neutralization bias to sera suggests that NTD mutations could have a significant effect on so- SARS-CoV-2 exposure to antibody neutralization.
* Important message
bioRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.