
Photographer: Matt York / AP
Photographer: Matt York / AP
A new drug for Alzheimer’s disease is on a fruitful path toward the U.S. market, and while many patients would take a license as a key advancement, it could also rekindle concerns about the scientific integrity of regulators.
Biogen Inc. said. Friday that the U.S. Food and Drug Administration expanded its review of aducanumab, an experimental drug that patients and their families see as a potential lifestyle. But the group ‘s history of cherry – raising efficacy data on the drug reveals a five – year – old son decision to support treatment for childhood muscle wasting disease, one that remains controversial.
The FDA watchdog and its own staff are concerned that the regulator and its leadership could develop a pattern of approving drugs of questionable value for disastrous conditions due to public pressure. Quickly approving Covid-19 medications such as hydroxychloroquine that was hit by former President Donald Trump and was subsequently deemed ineffective increases the anxiety.
“No one, except perhaps shareholders, will benefit from getting a product on the market that doesn’t work,” Caleb Alexander, a Johns Hopkins University epidemiologist and FDA consultant who does not think there is any available evidence supporting the Biogen drug, said in an interview.
Read More: Trump-Touted Plasma Therapy shows no benefit, researchers say
Both the FDA and Biogen declined to consider the interaction.
Investor interest was piqued on Friday when the group delayed a three-month decision until June 7 on whether the drug should be cleared, suggesting Biogen’s claim is receiving further consideration. Biogen and partner Eisai Co. said. in a report that they gave more to the group analysiss and data that will take some time to review.
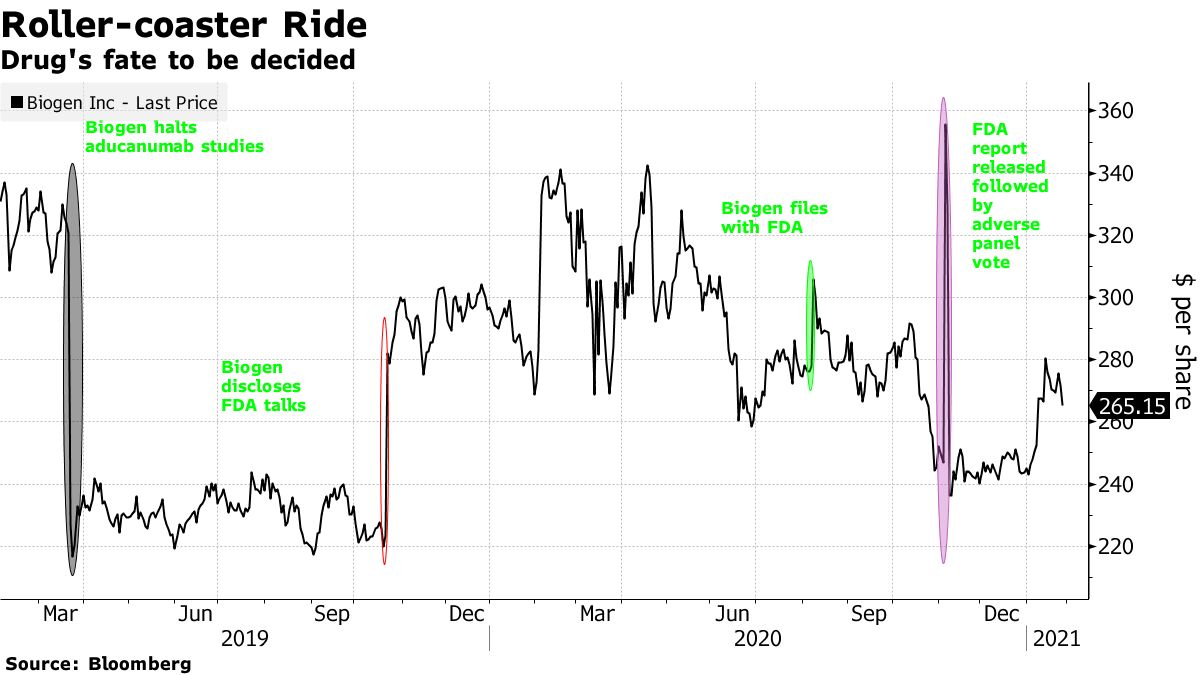
While the news met with a mixed response from analysts, Biogen shares rose 5.5% on Friday. They have been on a roller-coaster ride since early 2019 when Biogen, based in Cambridge, Massachusetts, stopped clinical trials of aducanumab because it did not work in patient trials. The stock went up later the same year when a reanalysis of one of the stop tests showed a halt of success.
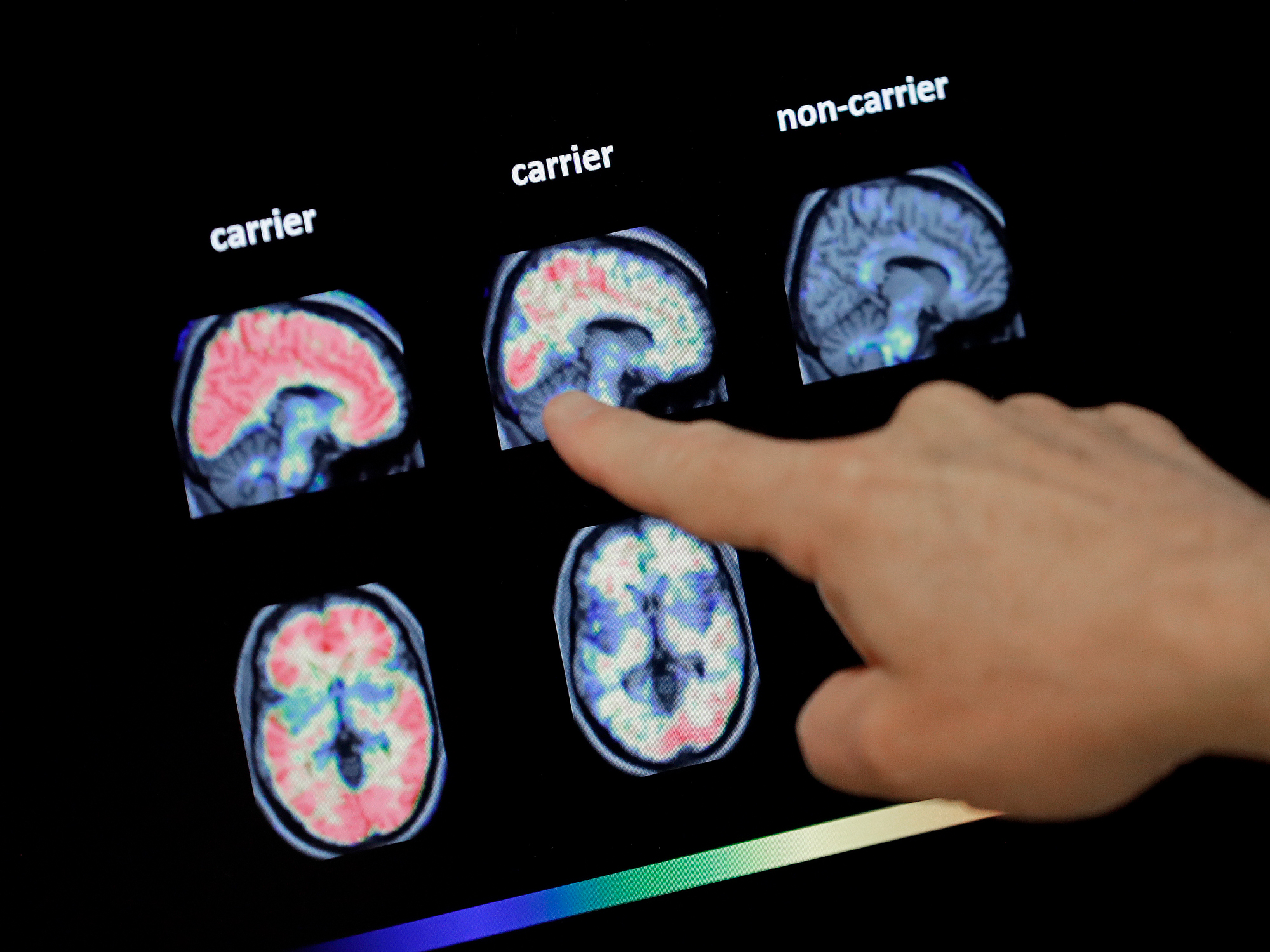
Aducanumab removes excess protein deposits called amyloid plaques from the brains of people with Alzheimer’s. It is intended to be used years in advance of possible symptoms, aiming at delay patient decline.
Recovery Hope
But there has been debate over whether plaque removal is of any benefit. Attempts to develop similar drugs have failed, although aducanumab and potentially promising data from an experiment there is a drug from Eli Lilly & Co. on some revivals hope.
As the U.S. population ages and Alzheimer’s rates go up, effective treatment would be a major setback. The 2019 reanalysis prompted Biogen to seek FDA guidance, leading to a all year round collaborated to further analyze data from the two trials, according to a document prepared by the group and Biogen ahead of a November meeting of group advisors.
“Given the unmet medical need and the unique nature of the data,” the regulator met four times with Biogen between June 2019 and June 2020 to discuss the studies, the document said. During the last meeting, they discussed Biogen’s ability to apply for aducanumab approval.
Billy Dunn, executive director of the Office of Neuroscience at the FDA’s Office of New Drugs, worked with the company on a November document evaluating the drug. The regulator and pharmaceutical companies typically write separate preparations for drug data ahead of advisory panel meetings, with the FDA taking an urgent look.
“Our group has been testing for five decades prior to FDA consultation meetings,” they said Michael Carome, director of the health research group Public Citizen, the government’s watchdog group. “We have never seen such a preparatory document.”
The FDA refused to provide Dunn for an interview.
When consultants outside the FDA met in November to discuss recommending the drug for approval, agency staff made the report clear to them. Evidence from the clinical trial was relatively positive “strong and extremely strong,” the document said. They dismissed unfavorable results from a second trial that was actually designed, saying they did not show the drug was ineffective.
Read More: Biogen Surges After FDA Reviews Support Alzheimer’s Drug
FDA statistician Tristan Massie disagreed. “Excluding data from a major test without sufficient justification is ignorant, statistically inappropriate and misleading,” he said in a presentation to advisory panel members.

Slip from presentation Tristan Massie, FDA statistician prepared for November 6, 2020 meeting of group advisors to discuss aducanumab.
But Massie was not given much time to give her views at the November meeting, as questions were directed to the biotech scientists.
Group reimbursed
“I thought the most surprising thing was when one of the panellists asked a statistical question and Dunn responded by saying, ‘You know, I’m not a statistician but maybe he could Biogen responded, ‘”said Brian Skorney, man Robert W. Baird Analyst & Co. to watch the meeting.
The panel’s discussion and recommendation against aducanumab was “a shocking and near-unanimous repetition” of the drug, wrote Biogen and FDA clinical reviewers, Cowen analyst Phil Nadeau at the time. But the drug could still be cleared. The group does not have to follow the consultants’ recommendations.
Biogen declined to say whether he submitted data to the FDA from another a trial, called Embark, which began in March 2020. The study is not the most rigorous form of clinical studies because it does not include a comparison group of patients who received a placebo.
Approximately 5.8 million Americans live with Alzheimer’s dementia, and the number is expected to nearly balloon 14 million by 2050, according to the Alzheimer’s Society. Patients and their families look for anything that could stop the situation’s brutal progression.

Photographer: Patricia Lake / UsAgainstAlzheimer’s
“If you had a 50/50 chance that this drug would work for me, it’s better than the zero chance I have today,” said George Vradenburg, co-founder Us Against Alzheimer’s, a patient advocacy group.
Vradenburg has seen two relatives on the side of his wife’s family die from Alzheimer’s. Any success for aducanumab could encourage investment and further research, possibly discouraging new therapies, he said.
Carome understands the despair; his mother died more than a decade ago of Alzheimer’s after battling the disease for 10 years. However, he is concerned about the FDA’s possible approval of a drug that is not working.
“It simply came to our notice then hope to millions of patients and their families, ”he said. “It could have a negative financial impact and could disrupt the Medicare program and, ultimately, could delay and weaken future research on Alzheimer’s drugs that could be effective. ”
The FDA’s treatment of the situation is similar to a program in 2016 when, over the complaints of its own reviewers, the leadership of a drug group for Duchenne muscular dystrophy, a fatal condition that largely affects young boys. Sarepta Therapeutics Inc. had only limited data. – and, for FDA advisors and reviewers, unbelievable – data on the drug’s effectiveness. But the drug, called Exondys 51, was backed by desperate parents whose sons were suffering.
Scintilla of effect
At the time, FDA reviewer Ellis Unger warned that the decision could set a precedent. Future drug licensing may be based on “just a side effect,” he said, according to agency documents posted online.

Tom Williams / CQ-Roll calling group
Dunn, then director of the department of neurology products at the FDA’s Center for Drug Assessment and Research, told a panel of group advisers convened in April 2016 that the group’s staff had “serious concerns” about data behind Exondys 51. But he and Unger were overtaken by Janet Woodcock of the FDA, who was named acting commissioner by President Joe Biden.
Woodcock declined to comment. Sarepta did not respond when he was reached for comment.
Now the group needs to figure out how to detect a potential standoff on aducanumab. Massie and other critics of the drug have suggested that a third, pre-FDA testing decision should clear up. Advocates want access for patients now. They say Biogen could investigate after the drug hit the market to try to prove the benefit of aducanumab.
Agreeing with the dangers of drugs sending a message that the FDA is willing to work on weak evidence, said Alexander, the Johns Hopkins-based FDA consultant.
“It is clear that this is a terrible disease where new treatments are urgently needed,” he said. “For that reason it is vital that the FDA and this market get it right. ”