The COVID-19 outbreak is still going strong one year after the novel coronavirus started in Wuhan, China, with the virus entering more infectious variants. A rapid and accurate virus detection strategy is very important to reduce the transmission rate of coronavirus syndrome 2 (SARS-CoV-2), the pathogen responsible for COVID-19. The global scientific research community has responded to this unique demand in diagnostic experiments by developing several detection platforms, the most sensitive of which are the detection of the viral RNA.
The current gold standard of testing – reverse transcriptase-polymerase (RT-PCR) chain reaction – is a 2-step assay that takes more than 60 minutes to validate a single sample. Reverse transcriptase is an enzyme that converts viral RNA to DNA (cDNA), which takes nearly 30 minutes. The cDNA is then amplified using quantitative PCR (qPCR), and the expanded DNA is detected with the help of fluorescent dye, which takes about an hour.
Reducing detection times and increasing sample size are crucial in reducing the spread of the virus. The research community has considered a number of new approaches that will help reduce assessment times for SARS-CoV-2 detection over one year.
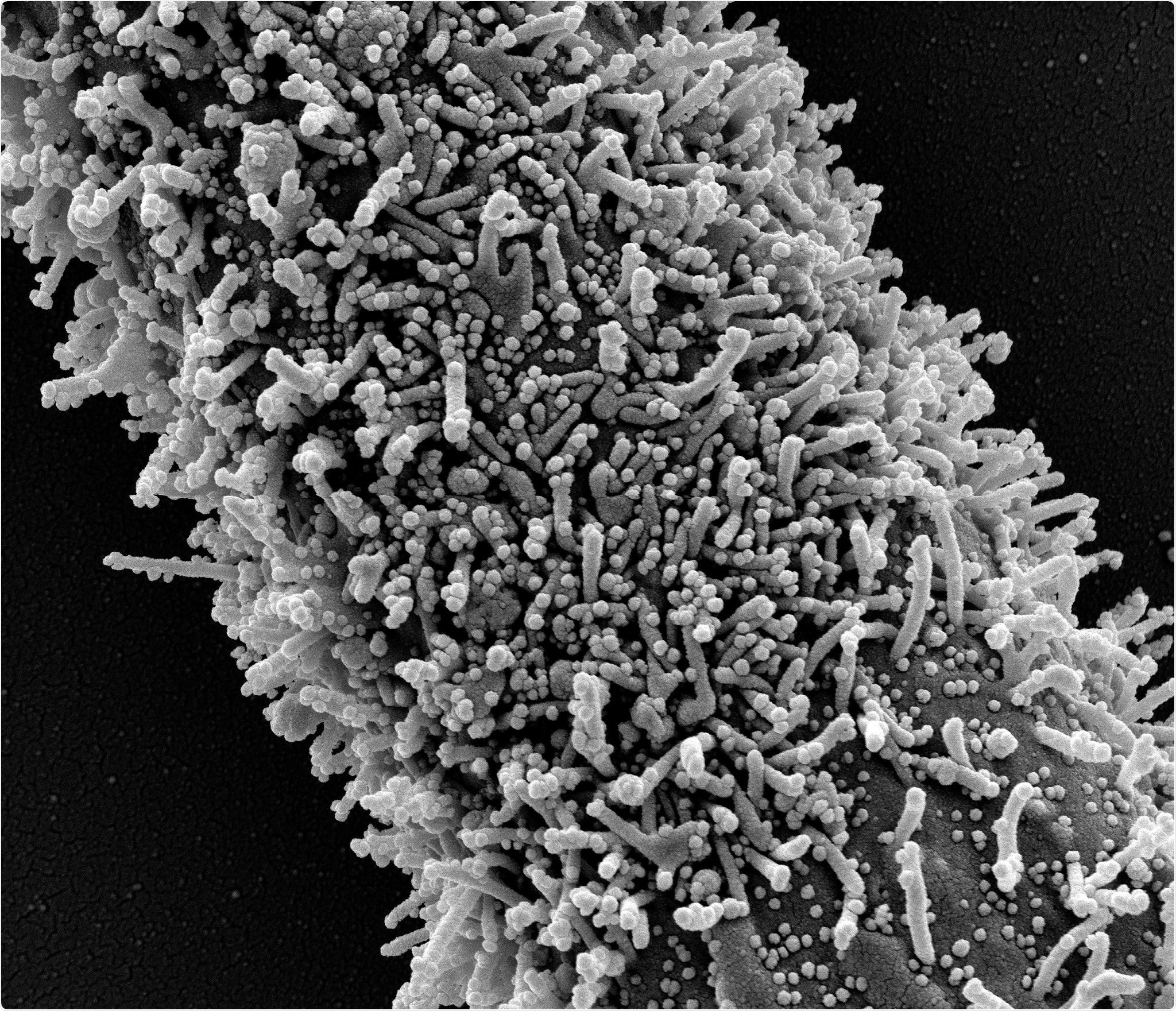
In one long CCL-81 cell with severe infection with SARS-CoV-2 virus granules. The small spherical structures in the image are SARS-CoV-2 virus granules. The protrusions are rod-shaped cells from the progenitor cells or pseudopodium. Image captured at the NIAID Integrated Search Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
Restore isothermal SARS-CoV-2 detection without transcriptase
Recently medRxiv* preprint research paper, researchers from the UK reported a rapid isothermal method for the detection of SARS-CoV-2. The researchers used a new method to convert RNA to cDNA without using the reverse transcriptase enzyme. This new mechanism rapidly enhances the DNA by using the exponential extension response (EXPAR).
Since the new reverse transcriptase-free (RTF-EXPAR) approach combines the conversion of RNA to DNA and amplification of the cDNA using EXPAR in a single step, it can detect SARS-CoV-2 in a sample in less than 5 minutes.
How does the EXPAR approach work?
In the EXPAR method, cDNA expansion occurs at a single temperature, which avoids time-consuming heating and cooling steps. Also, the amplicon is relatively small – typically around 15–20 centers in length – compared to both PCR and isothermal loop-centric AMPlification (LAMP) methods. Thus, once stimulated, EXPAR can produce up to 108 strands of DNA in a matter of minutes. Similar to the RT-PCR assay COVID-19, colon formation in EXPAR is monitored spectroscopically with the help of fluorescent interferometric dyes such as SYBR Green.
“Identifying sequences of optimal nucleotide in the target genome is an important element of developing a successful EXPAR assay.”
The researchers used this EXPAR approach in a two-step process. First, they performed enzymatic digestion at 50 ° C for 5 min of DNA Binder (1 µM) in the presence of a SNA-CoV-2 RNA patient sample (72.7 copies / µL). They then added this solution to the EXPAR reagent mixture to increase the obtained DNA. Played in triples, this step offered an extension time of 3.17 ± 0.24 minutes.
An RTF-EXPAR assay could be modified for use in detecting other RNA viruses
To summarize, the researchers used a new transversease-free isothermal amplification method anonymous RTF-EXPAR to confirm successful detection of SARS-CoV-2 RNA with a total assay time under 5 min. This approach is not only significantly faster than the RT-PCR assay, which requires at least 60 minutes to test one sample but also performs better than LAMP and late-flow antigen methods.
“Not only is this time much faster than RT-PCR (assessment time at least 60 minutes) but it also performs better than LAMP and 30-minute lateral flow antigen tests in conventional use. . ”
According to the authors, RTF-EXPAR should be fully compatible and ready for use on the same equipment currently used for COVID-19 RT-PCR evaluations. In addition, the speed and simplicity of the essay make it possible to customize this method for use in the detection of a range of other infectious diseases caused by RNA-based viruses such as Ebola and RSV. .
“In conclusion, through the use of a new transversease-free isothermal amplification method, RTF-EXPAR, incorporating a selective DNA-binding endonuclease, we have demonstrated the successful RNA of SARS-CoV-2 the total assessment time is less. the 5 minutes. ”
* Important message
medRxiv publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be seen as final, guiding health-related clinical practice / behavior, or be treated as information established.
Magazine Reference:
- Sub-5-min validation of SARS-CoV-2 RNA using Reverse Transcriptase-Free Exponential Amplification Reaction, RTF-EXPAR, Jake G. Carter, Lorea Orueta Iturbe, Jean-Louis HA Duprey, Ian R. Carter, Craig D Ed., Marium Rana, Andrew Bosworth, Andrew D. Beggs, Matthew R. Hicks, James HR Tucker, Timothy R. Dafforn, medRxiv 2020.12.31.20248236; doi: https://doi.org/10.1101/2020.12.31.20248236, https://www.medrxiv.org/content/10.1101/2020.12.31.20248236v1