The Pfizer vaccine was the first to gain emergency approval from the U.S. FDA and vaccines began this week.
Photographer: Bing Guan / Bloomberg
Photographer: Bing Guan / Bloomberg
The first sensory reaction known to the Pfizer Inc. vaccine has been reported. Covid-19 in the U.S. in Alaska as some reptiles began to appear in an effort to spread the pictures across the country, highlighting the daunting challenges we face for a historic vaccination campaign.
A health care worker in Juneau, Alaska, reported no history of allergies and shortness of breath 10 minutes after being shot, the state health department said in a reported Wednesday.
The man was admithe was taken to the emergency room and given Pepcid, Benadryl and epinephrine via an intravenous drip. The patient stayed overnight and is in stable condition, according to the Alaska Department of Health.
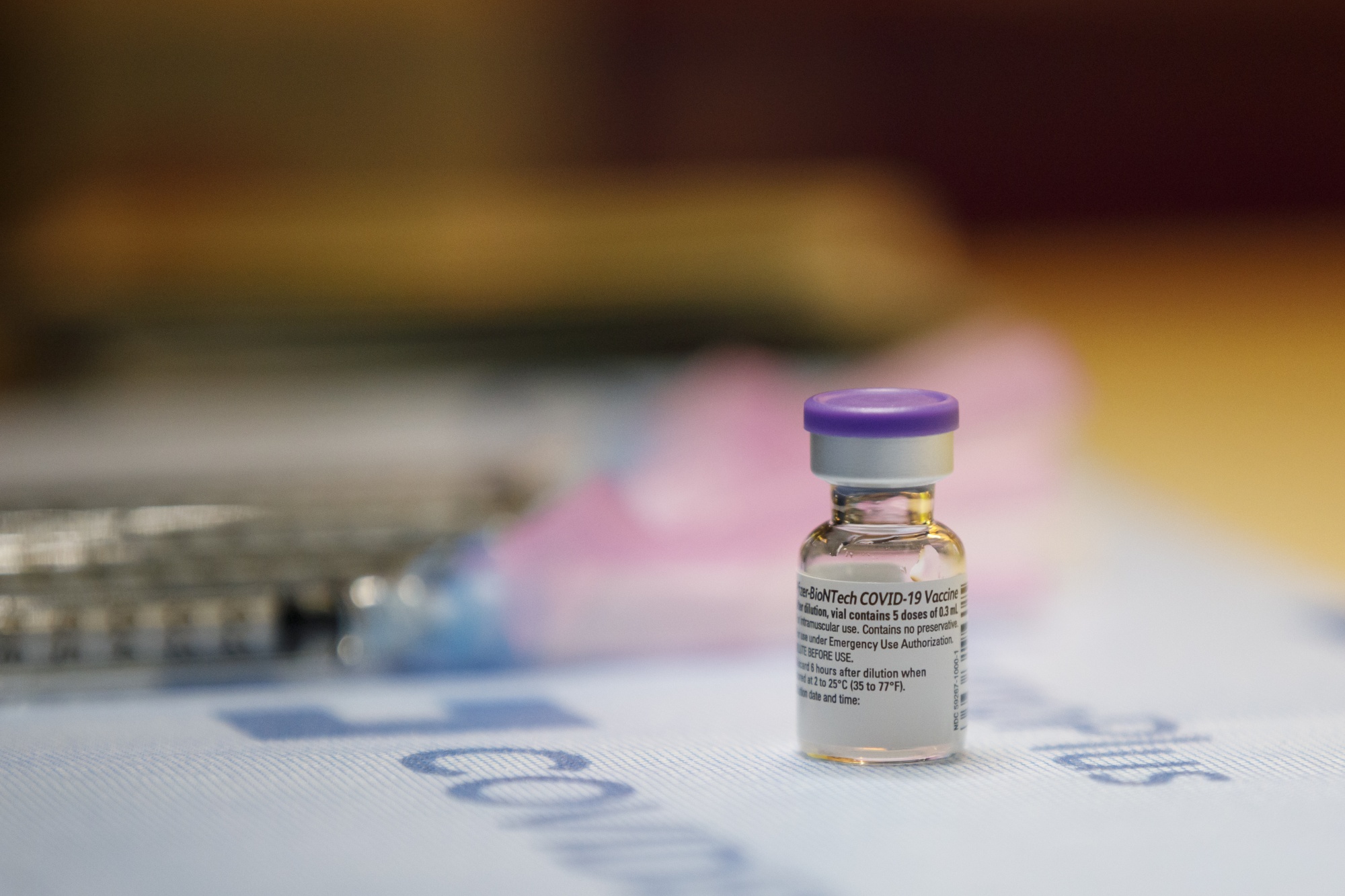
Vial of the Pfizer-BioNTech Covid-19 vaccine.
Photographer: Cole Burston / Bloomberg
The program follows reports of a number of similar reactions in the UK, where health officials advised that anyone with a history of severe allergies should not take the Pfizer vaccine. U.S. regulators last week recommended that people should be monitored for allergy symptoms for 15 minutes after receiving the bullet, which was approved for emergency use by the Food and Drug Administration on Friday.
Pfizer coordinates with local officials, closely monitors all reports suggesting a severe allergic reaction and updates billing language if needed, spokeswoman Jerica said Pitts in a statement.
The incident came to light when the first hiccups in the U.S. vaccine circulation erupted on Wednesday, including a holdup in the delivery of 3,900 beats to two states and the news that Pfizer would deliver about 900,000 more fewer doses next week than expected to be scrapped this week.
With the release of the Pfizer bullet, a major effort began to move. Vaccines from two company production facilities in the Midwest are being sent to all 50 states, the first wave in a month-long campaign to protect as many people as possible and end a deadly pandemic. over 300,000 in the USA A similar image made by Moderna Inc. is expected to acquire US approval in the coming days.
One challenge in moving and storing Pfizer’s vision, which it developed with the German drugmaker BioNTech SE, is required to be maintained at ultracold temperature in special freezers and containers. Four delivery luggage of the photograph was withdrawn from delivery to California and Alabama this week and returned to the company because they were colder than expected, according to Gustave Perna, the army’s general-in-chief. serve as chief operating officer of Operation Warp Speed.
Each of these accessories can be used to vaccinate 975 people. Pfizer has stated that its formula must be stored at 70 degrees below zero Celsius, equivalent to minus 94 degrees Fahrenheit. These belongings were found much colder, according to Perna.
“We were not taking any chances,” he said at a press conference on Wednesday. Pfizer and federal health agencies are working to see if the formula can still be used when it reaches such low temperatures, according to Perna.
Pfizer uses the doses in temperature-controlled shipping vessels developed by its own engineers. Each device is equipped with GPS trackers “for continuous and real-time monitoring and temperature,” according to the company, which has a round-the-clock control tower that monitors all loads.
Less satisfaction
U.S. officials also said at the meeting that about 2 million doses of the Pfizer vaccine will be dispensed for the country next week, less than the 2.9 million available this week when the first pictures are taken. sent away. They gave no explanation.
Alex Azar, secretary of the Department of Health and Human Services, acknowledged product challenges previously announced by Pfizer. “As you know, they ended up coming short with half of what they thought they would be able to achieve and what they announced they would be able to achieve” in 2020 , Azar said.
Operation Warp Speed has said it expects enough vaccines between Pfizer and Moderna to contain 20 million Americans in December. He has contracted for 100 million doses of the Pfizer vaccine, which requires two shots per patient. Azar and other officials pushed back reports recently that the U.S. government refused to buy more doses from Pfizer, saying the company could not guarantee that they would be ready by mid-2021.
“They are currently working to their full potential to deliver the first 100 million of the contract, and we are providing manufacturing support,” he said.
Pfizer spokeswoman Sharon Castillo said in an email that the company had not experienced any production issues.
Unlike other vaccine developers supported by Operation Warp Speed, Pfizer did not receive research and development funding from the U.S. government. Azar said that means officials didn’t have as much insight into the company’s production.
“The relationship Pfizer wanted with Operation Warp Speed is the guaranteed purchase of a vaccine if it has been approved by the FDA,” he said. Azar said the government is talking to the company about getting more information about any challenges they face.
Unused antibodies
Azar and other officials also said that promising monoclonal antibody therapies authorized for Covid patients who do not need to use a hospital are being used as often as they had hoped.
The drugs, manufactured by Eli Lilly and Co. and Regeneron Pharmaceuticals Inc., is available for emergency use for less serious cases. They must be delivered through inclusion, which has created supply challenges for medical providers.
“We really are hope to see a much higher level of uptake of the doses delivered to the healthcare facilities, ”Moncef Slaoui, chief consultant of Operation Warp Speed, told the meeting.
The antibody drugs are intended for elderly patients and people with other diseases that put them at greater risk of hospitalization from Covid-19. They are most effective when delivered early.
“The best time to get these antibodies is not when you show up in the emergency room, but shortly after you are diagnosed,” Azar said. The U.S. has dispensed 300,000 doses to states, and many states have supplies, he said.
– Supported by Riley Griffin, and Jacquie Lee