
Editor’s note: Discover the latest COVID-19 news and instructions in the Medscape Coronavirus Resource Center.
If ever there was evidence of the power of the scientific method, it is this: almost a year after SARS-CoV-2 hit our shores, we have two vaccines authorized by the U.S. Food and Drug Administration to prevent COVID-19. And from the looks of it, another one is on the way.
Johnson & Johnson (J&J) on Friday announced the main results of the ENSEMBLE trial, which examined the effectiveness of the COVID-19 vaccine, raising an overall efficacy rate of 66%.
While the 90% to 95% efficacy has not been reported for the Pfizer and Moderna vaccines, 66% is certainly not going to sneeze at all. In fact, a single – dose vaccine that can stay in the refrigerator for 2 months is definitely a play change. While the United States has sufficient infrastructure to control the ultracold conditions required for the Pfizer vaccine and the extreme cold conditions required for Moderna, many countries that do not have this capability do not have this capability. so developed. In addition, a “one-size-fits-all” approach significantly increases the potential of mobile clinics, vaccine fairs, and outreach to rural people.
To understand the impact of this third coronavirus vaccine option, I want to compare it with the two that exist across several areas: equipment, efficacy, side effects, and the likelihood of the vaccine escaping. But a caveat here: I only work from a press release from J&J. This data has not been peer-reviewed, and there are many dark corners that we cannot examine until we have more information.
Action equipment
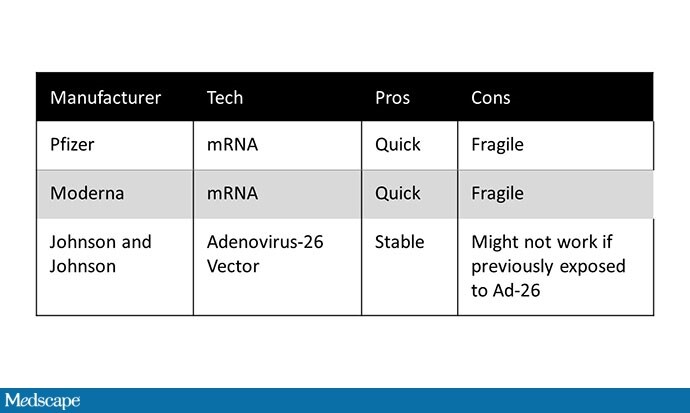
The Pfizer and Moderna vaccines are based on mRNA technology – well established in research, but have never been used on such a large scale. The mRNA directs human cells to produce the spike protein of the coronavirus, which binds the virus to cells, producing antibodies.
The benefit of mRNA vaccines is the speed with which they can be developed. Downside? MRNA is fragile, tending to break down at room temperature into sugar-free and acid-free pieces.
The J&J vaccine is not your standard vaccine, either, depending on adenovirus vector technology. Again, this technology is not new, but this is its first comprehensive test. The vaccine is a modified version of adenovirus 26; a specific gene was removed to ensure that it cannot reproduce in human cells. Instead, the gene for the SARS-CoV-2 spike protein is inserted. This complex is grown in a pool of cells engineered to express the missing gene, allowing the modified adenovirus to reproduce. The virus is then cleared and absorbed – spike proteins and all – and produces a strong immune response.
The upside of this approach is its sustainability; there are no ultracold storage terms here. One theoretical disadvantage? Adenovirus 26 is a virus that some of us may have – it is a cause of conjunctivitis – and it is not yet clear whether prior infection with adenovirus 26 can reduce the effects of the vaccine.
Efficiency
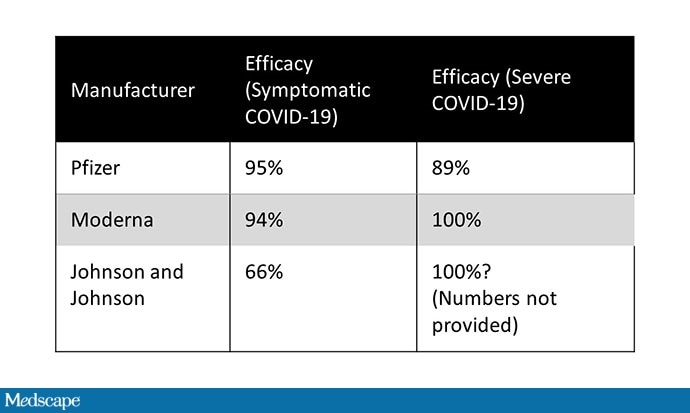
The initial efficacy of mRNA vaccines was the highest level of coronavirus news in 2020. Vaccines with efficacy greater than 90% are very rare; for two to be in so little time have completed a miracle. What, then, do you make of the 66% reported efficacy for the J&J vaccine?
More importantly, the vaccine efficacy numbers you hear are based on prevention symbolic COVID-19, not true COVID-19. Vaccines are not just benefits. The real benefits come from reduced hospitalization, deaths, and increased virus spread. We do not know about the potential of available vaccines to reduce viral transmission, but we do have data on clinically critical hospital outcomes and mortality.
The Pfizer vaccine is 89% effective in preventing death and hospitalization. Moderna – 100%. J&J reports that there is “complete” hospital protection and death 28 days after vaccination, meaning (without giving us the raw numbers) that such incidents did not occur in the group. vaccine at that time. In particular J&J did not report such events in the placebo group, which would greatly help to drive the meaning of these results home.
However, it is clear that even a vaccine that is imperfect for preventing all symptoms could be excellent at preventing hospitalization and death – and that is a widespread benefit.
Summary: If hospitalization and death are so important, why have they not been used as key outcomes in vaccine trials? Expediency. Hospitals and deaths are (fortunately) among those with COVID-19. So we would wait longer until enough of these results occur before we could estimate the effectiveness of the vaccine. The use of symbolic infection is a well-tolerated symptom – the idea is that if a vaccine reduces symptoms, it is certain to reduce hospitalization and death. So far, the data has confirmed that assertion.
Even if we focus on symbolic infection, it is not entirely correct to compare the efficacy of the J&J vaccine with that of the mRNA vaccines. There are lots of apples and songs here. First, the J&J vaccine is a one-dose vaccine; if patients received an increase in the J&J vaccine, efficacy would increase. A single dose of the Pfizer or Moderna vaccines appears to provide around 80% efficacy, according to the data we have.
Another key issue is time. The mRNA vaccines were studied earlier in the pandemic, before rampant diseases caused a number of potentially immunosuppressive changes. In fact, we can attribute almost the overall underperformance of the J&J vaccine in South Africa to the B.1.351 variant that is endemic there.
Side effects
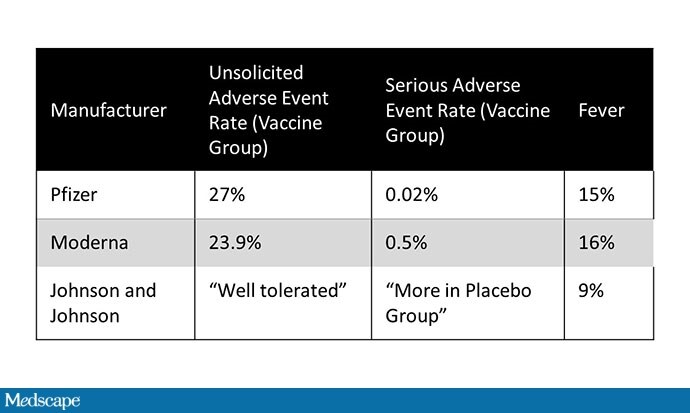
J&J did not release much data on adverse events other than reporting that the vaccine was “very tolerable,” there were no cases of anaphylaxis, and adverse events were more common in the placebo group compared to the vaccine. vaccine group. All good news, I believe – but very few hard numbers. J&J reports a fever rate of 9%. You can compare that with a rate of about 15% after the second dose of the mRNA vaccines.
Vaccine escape
Despite breathless news stories of all new coronavirus variants, it is worth noting that SARS-CoV-2 is not a fast-moving virus. By flu standards, this coronavirus is a strong maintainer. But hundreds of millions of diseases worldwide have provided hundreds of millions of opportunities for new mutations, some of which may be able to bypass the immune sensitivity that vaccines create. .
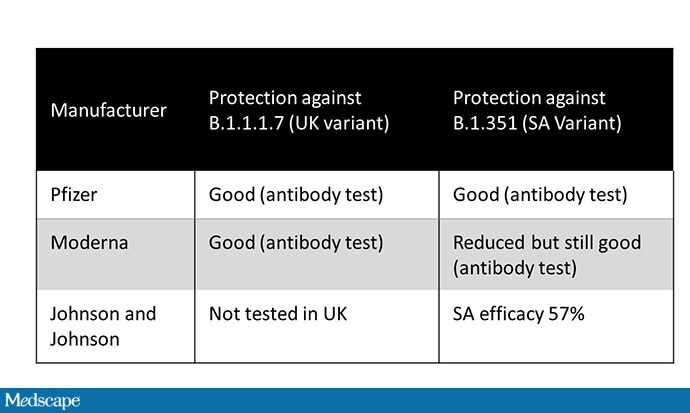
To date, there are two databases that allow us to assess whether different vaccines can escape. The first uses serum from people with vaccines. These neutralization assays ask whether the antibodies in that serum keep the virus from growing in culture. So far, both Pfizer and Moderna are showing good results against the British version B.1.17. The Pfizer vaccine performs slightly better against the South African variety, and Moderna slightly worse, although it is still effective. These estimates may underestimate true efficacy, as only the immunity of antibodies has been evaluated, whereas vaccines lead to low (antibody – based) and cellular immunity.
The other way to find out if a vaccine protects against a variable is to monitor the variable infection rate among people who have been vaccinated. Given that Pfizer and Moderna test data came out before the variables were identified, we cannot speak to this – although there is no doubt that they are still collecting that data on their partners. J&J data, on the other hand, suggest reduced efficacy – the protective effect in South Africa (where 95% of diseases were due to B.1.351) was only 57%.
Ultimately, we have to accept that changes will occur, and it will be difficult to determine which vaccine may be so effective against any particular variant. My hope is that all vaccines provide at least some protection against alterations; converting a deadly COVID-19 to a severe cold or flu is a good recommendation.
In addition, we should expect that we will need a vaccine update to account for new strains, as we currently have for influenza. The mRNA vaccines have an advantage here because a direct update requires the addition of the new series to the production line.
What to do
In a short time we will have three vaccines with different side effects and side effects. Although supply is scarce, the decision on which vaccine to take is simple. Anyone who is eligible for a vaccine should receive anything they receive.
Soon, however, there may be several options available, and I will give the following guidance. Where there is a choice, the highly effective mRNA vaccines should be given to those at risk of death from COVID-19: the elderly and those with certain preexisting conditions. The J&J vaccine may be particularly suitable for those who are at high risk for exposure to COVID-19 but who are still at lower risk for adverse outcomes: consider frontline and essential workers.
This calculus may change when we know how these vaccines may (or may not) prevent asymptomatic transmission, but for now, this sounds like a reasonable first approach. Of course, having a choice of vaccines is an extremely important problem to have.
Perry Wilson, MD, MSCE, is a nephrologist at Yale University and holds a master’s degree in clinical epidemiology. He is often selective about the design and interpretation of clinical studies, including his video series on Medscape, Impact Factor.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube.