U.S. regulators have approved the first long-term drug combo for HIV, a monthly supplement that can replace the daily pills now used to control infection with the AIDS virus .
Thursday’s agreement on the two-image combo called Cabenuva is expected to make it easier for people to stay on track with their HIV medications and do so with more privacy. This is a big change from not so long ago, when patients had to take several pills several times a day, carefully weighed around food.
“That will improve quality of life” to require treatment only once a month, said Dr. Steven Deeks, an HIV expert at the University of California, San Francisco, who has no links to the drug’s manufacturers. “People don’t want those everyday memories of being infected with HIV.”
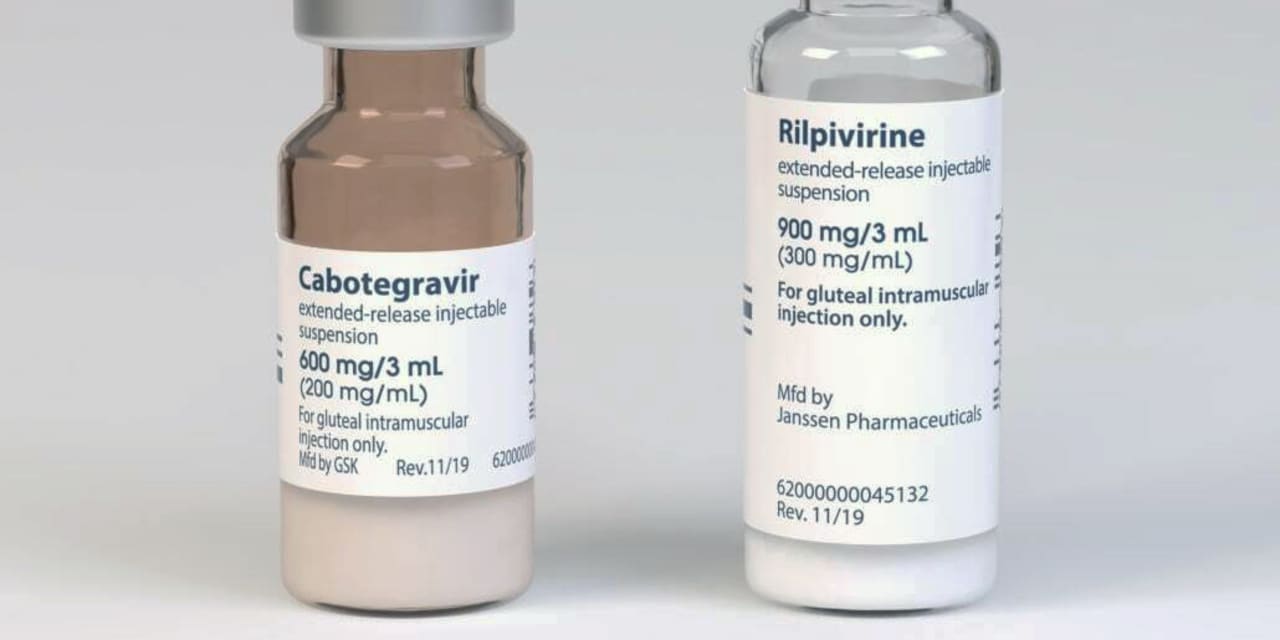
Cabenuva is a combination of rilpivirine, sold as an Edurant by Johnson & Johnson’s JNJ,
Janssen unit, and a new drug – cabotegravir, from ViiV Healthcare, owned mainly by GlaxoSmithKline GSK,
They pack them together and present them as separate pictures once a month. Doses are also tested every two months.
Cabenuva was approved by the U.S. Food and Drug Administration for use in adults who have had good control of their infection with conventional HIV medications and have not shown any signs of viral attack on both Cabenuva drugs.
The group also agreed to take a pill version of cabotegravir with rilpivarine for a month before moving on to the pictures to make sure the drugs are getting up well.
ViiV said the bullet combo would cost $ 5,940 for an initial dose, higher and $ 3,960 per month thereafter. The company said that is now “within range” of the cost of one-day pill combos. What a patient pays depends on insurance, income and other things.
Studies found that patients preferred the views.
“Even people who take one pill once a day just reported an improvement in quality of life switching to injection,” said Dr. Judith Currier, an HIV expert at the University of California, Los Angeles. She is consulting for ViiV and wrote a report accompanying one study of the drug in the New England Journal of Medicine.
Deeks said long-term perspectives also give hope to organizations that have a hard time sticking to treatment, including people with mental illness or substance abuse problems.
There is a “huge unfulfilled need” that could fill the pictures, he said.
Separately, ViiV intends to seek approval for cabotegravir for HIV prevention. Two recent studies found that bi-monthly cabotegravir shots were better than daily Truvada pills for keeping unprotected people from catching the virus from an infectious sex partner.