
Editor’s note: With the coronavirus vaccination effort now underway, you may have questions about what this means for you and your family. If you do, send them to The Conversation, and we’ll get a doctor or researcher to answer them. Here, Dr. Mona Hanna-Attisha, a public health pediatrician whose research highlights the water crisis of Flint, Michigan, answers questions about the vaccine and allergies, and when children may be vaccinated .
If I have allergies, should I still be vaccinated?
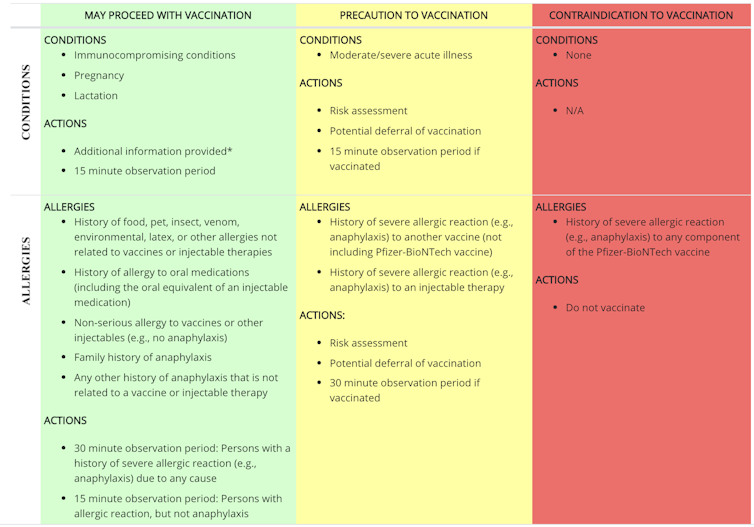
If you have a history of allergies to food, pets, insects or other things, the Centers for Disease Control and Prevention recommends that you go ahead with vaccinations, with observation time. If you have a history of a severe allergic reaction, or something called anaphylaxis, to another vaccine or injectable medication, your doctor may carry out a risk assessment, cancel your vaccination, or go ahead and then look after you. after immunization. The only reason to avoid a vaccine is a severe allergic reaction to any part of the COVID-19 vaccine. The CDC has specific recommendations for post-vaccination observation.
As the vaccine goes out to a wider population, how will adverse events be monitored?
The CDC and the Food and Drug Administration encourage the public to report potential adverse events to the Vaccine Adverse Event Reporting System, or VAERS. This national system collects this data to look for unforeseen adverse events, which are likely to occur more frequently than expected or which have unusual incident patterns. Anyone who has been adversely affected should report it to the system.
Reporting an adverse event is a crucial step in ensuring safety and to help the CDC monitor the vaccines. Safety is a top priority, and scientists and public health officials need to know about adverse effects.
The incidence of adverse effects differs mainly from the usual side effects of a vaccine. Vaccines can cause side effects, such as soreness at the injection site or redness. Harmful incidents are more dangerous and can sometimes be life threatening. If you are unsure whether you have been adversely affected or adversely affected, you can still report the incident.
Participants will be given an information sheet on receipt of the vaccine. Vaccinated health care providers must tell VAERS bad events after receiving the vaccine. In addition, under the terms of an emergency use permit, healthcare providers are also required to have revised safety reporting requirements that may arise.
The CDC is also implementing a new smartphone-based device called v-safe to monitor people’s health after receiving the COVID-19 vaccine. When you receive your vaccine, you should also receive an information sheet telling you how to register in v-safe. If you sign up, you will receive regular text messages directing you to reviews where you can report any problems or side effects you may have after receiving the COVID-19 vaccine.
CDC
When can children under 16 be vaccinated?
It is likely to be several months. The currently authorized Pfizer vaccine and the soon-to-be-authorized Moderna vaccine do not apply to children. More research and clinical trials are needed to include younger children in COVID-19 vaccine trials.
According to the American Academy of Pediatrics, Pfizer has enrolled children down to the age of 12 and applied for an emergency use permit for vaccines down to the age of 16. Moderna, which expects its vaccine to receive an emergency use license from FDA any day, about to start a similar study.
In the United Kingdom, AstraZeneca is allowed to enroll children ages 5 to 12 in clinical trials, but the pharmaceutical company has not yet enrolled any children in trials in the US.