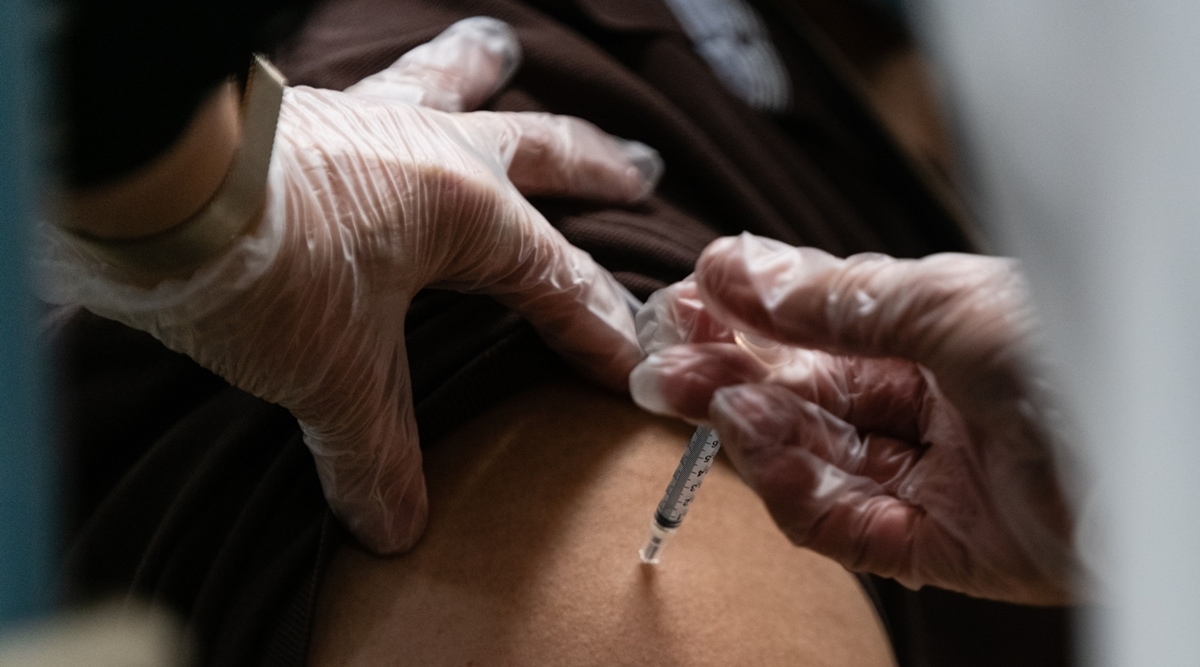
After months of waiting, people in several countries have started getting the vaccine for coronavirus. In Delhi, for example, Prime Minister Arvind Kejriwal announced that recently 51 pictures of vaccination will be given to lakh people.
Vaccines take years of testing and testing. In a series of explanations, the World Health Organization (WHO) analyzed how vaccines are developed, stored and then dispensed.
What is a vaccine?
Simply put, WHO in his article has “tiny particles of the disease-causing organism or the bluetongue for making the tiny particles.” Along with this, there are also other ingredients – all of which are tested for safety in the manufacturing process – to keep the vaccines safe and effective. These include antigen, preservatives, stabilizers, surfactants, residues, diluent, and adjuvants. These ingredients are mostly those that have been used in many other vaccines for decades in billions of doses.
 Vaccines take years of testing and testing. (representative, file)
Vaccines take years of testing and testing. (representative, file)
How are vaccines developed?
All vaccines undergo extensive and rigorous testing and screening and assessments to determine which antigen should be used to induce an immune response. This does not include testing on humans but on animals. If the vaccine stimulates an immune response, it is then tested in human clinical trials in three stages, explains WHO.
In the first stage, a selected number of volunteers receive the vaccine to determine if it is generating an immune response and to determine the correct dosage. In the second stage, it is then given to hundreds of volunteers. This phase involves several trials to assess the effect of different forms of the vaccine on different age groups. This involves another group not receiving the vaccine to compare the results and understanding whether the changes in the vaccine group have been caused by chance or because of the vaccine.
Finally, in the third stage, the vaccine is given to an even larger group of volunteers and compared to a group of people who do not receive the vaccine. This level is usually done across several countries and sites within a country.
Once the results of these clinical trials are available, officials in each country will closely review the data and then decide whether the vaccine can be approved for use. “Vaccines need to be proven to be safe and effective over a wide population before they can be adopted and included in a national immunization program. The bar for vaccine safety and efficacy is very high, recognizing that vaccines are given to people who are otherwise healthy and particularly free from the disease, ”read a WHO article. Further screening will be carried out once the vaccine is introduced.
How is it packaged?
The WHO explains that once the vaccine is made in large quantities, it is stored in glass filters and then carefully packaged for cold storage and safe transport. The packaging is made in such a way as to ensure that it is able to withstand extreme temperatures and also other dangers associated with being transported worldwide. Most vaccines are stored between 2-8 degrees Celsius. The vaccine can be just as effective or as dangerous if it is too hot or cold. But some of the new vaccines are stored at -70 degrees Celsius.
How are vaccines given?
“Special equipment” is used to keep the vaccine safe. Once in the country of destination, refrigerated trucks transport the vaccine from the airport to the cold room of the warehouse. It is then transported to regional centers in portable ice boxes. New technologies have created some portable devices that keep vaccines at the cold temperature for several days without the need for electricity, ”adds WHO.
For more lifestyle news, follow us on Twitter: lifestyle_ie | Facebook: IE Lifestyle | Instagram: ie_lifestyle