The New York TimesFeb 05, 2021 13:12:55 IST
Contrary to the possibility of possible coronavirus changes by avoiding routine vaccines, treatments and tests for the virus, the Food and Drug Administration is preparing a plan for action in the next few weeks. ahead. To date the Pfizer and Moderna vaccines have been effective against known variants of the coronavirus, but are less potent against the variant first identified in South Africa. This variation has only been confirmed in three states in the United States so far, but the country ‘s vigilance is sparse and other issues may be missed.
On Thursday, all but seven of the 618 cases of coronavirus variants identified in the United States to date have been implicated in a rapid variant first observed in Britain, according to data from the Centers for Disease Control and Prevention.
“If unforeseen changes reveal that vaccines are ineffective, we need to change quickly,” Janet Woodcock, the group ‘s acting director, said in a statement to reporters on Thursday. New versions of the vaccine needed to be produced quickly, tested and disseminated.
Officials at Pfizer and Moderna have said they are willing to roll out their vaccines as needed and that the process could be completed in as little as six weeks.
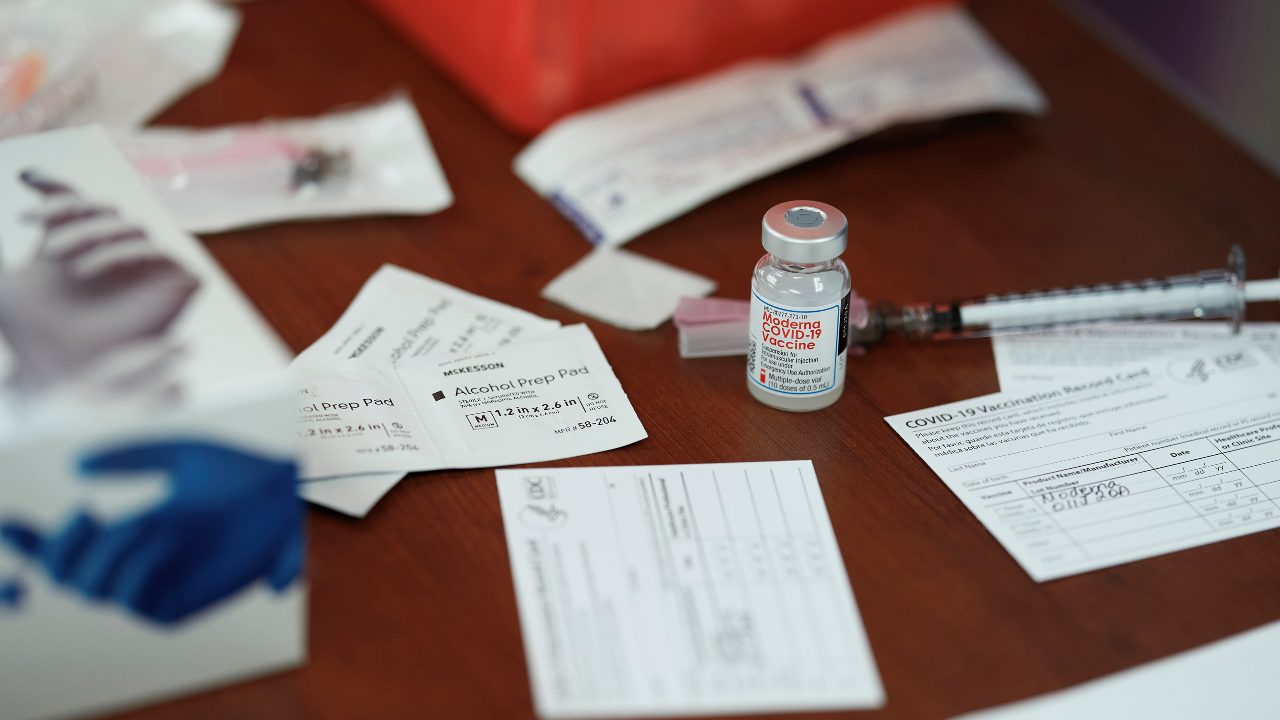
Vial of Moderna COVID-19 vaccine in Mount Pleasant, Texas, on December 21, 2020. Lack of images for yellow fever, polio and other diseases has resulted in ingenious solutions even in very poor countries. (Cooper Neill / The New York Times)
Dr Woodcock declined to disclose any information on how the FDA intends to evaluate the cut vaccines, but said the group needed smaller and shorter tests than in the original tests run by Pfizer and Moderna.
If routine vaccines are ineffective against a new variant, “we cannot go through more than 30,000 patient tests,” she said. “There are a few scarcity of comprehensive efficacy tests that we can use to move or possibly add components to existing vaccines.”
Dr Woodcock said the plans will be released for comment from scientists before they are implemented. The group can also look to an advisory committee to help lead any changes to existing vaccines.
The FDA also plans to release guidance documents for monoclonal antibody treatments and for testing of the virus. The monoclonal antibody made by Eli Lilly and one of the two antibodies in the cocaine made by Regeneron are powerless against the variant circulating in South Africa, according to a recent study.
“We knew from the very beginning that monoclonals were vulnerable to this kind of strain movement,” she said.
Laboratory tests for the coronavirus that rely on a method called PCR seem to detect the new mutations correctly, but if that changed, they could easily change, the official said. Dr. Woodcock. However, it would be “much more complex” to determine whether antigen tests performed en masse are missing changes.
Apoorva Mandavilli. c.2021 New York Times Company