Three articles published in Nature describe studies of human prenatal development outside the body. The approaches used in the studies may shed light on emerging events as organ initiation.
A group from the Weizmann Institute of Science, Rehovot, Israel, grew mouse embryos halfway through their prenatal development, to the point where end organs were formed. A group from the University of Texas Southwestern Medical Center, Dallas, Texas, and a group from Monash University, Melbourne, Austrlia, created “blastocysts” similar to human blastocysts from gas cells. All three projects move the field forward significantly.
Understanding dance requires early development of an in vitro interface that models the complex connections between the developing embryo and the placenta. The studies, while covering different species, provide a glimpse of what remains a mysterious time of embryogenesis.
Jacob H. Hanna, MD, PhD, and colleagues at the Weizmann Institute of Science conducted a study introducing a rotating, static, rotating bottle culture platform with human cord blood serum and pressurized oxygen in the culture they were a natural 5-day mouse. embryos for a week. Heads, heartbeats, and hind legs appeared in the glass, as can be seen in a video posted on YouTube.
A variety of methods – molecular analysis, histology, and single-cell RNA-sequence to assess gene expression – confirm that all three layers of the embryos are “ex-utero”, as the body calls them, matching those of natural growth in vivo. Tests are possible. The researchers used green fluorescent protein to identify the neural cells of the ectoderm and red fluorescent protein, called tomato, to identify endoderm cells. They added viruses, toxins, other chemicals, and human cells to the developing mice. Each of these fertilizers allowed the team to see and measure different aspects of early development.
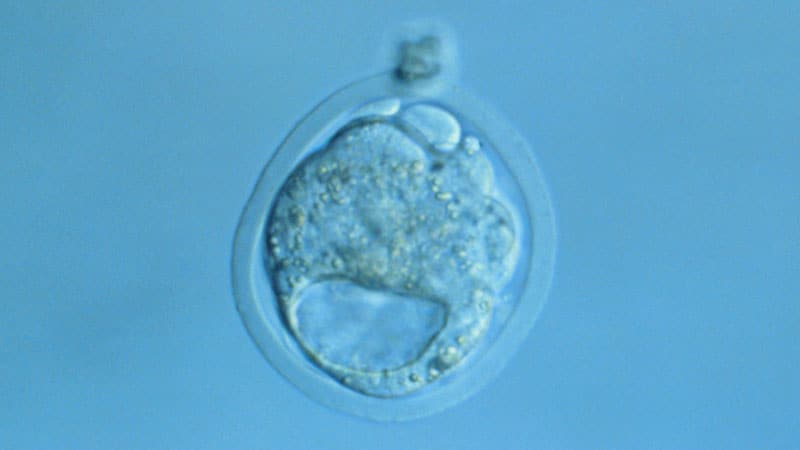
The human blastoid studies focused on the earliest part of the embryonic period, when there are only layers of cells in a sphere. The small cell balls resemble naturally formed blastocysts, the fluid-filled areas in which the outer cells (trophectoderm) cause external structures. A small accumulation of cells, called the epiblast, attaches to the interior of the globe and causes the embryo. Blastocysts have only three cell types, but the cells quickly divide and form layers that then interact and react as the organization takes place, starting through the Third week.
In their study, lead author Jun Wu, PhD, and the University of Texas group extracted “human blastoids” from human embryonic gas cells and from human pluripotent gas cells (iPS). The human embryonic stem cells have been licensed by the National Institutes of Health.
In the third study, lead author Jose Polo, PhD, from Monash University, and colleagues used iPS cells to create their “iBlastoids,”. Similar to the study performed in mice, the human blastoids mimic the exact contraction.
“They are similar to blastocysts in morphology, size, cell number, and all three cell types are organized in a blastocyst-like manner,” Wu, of the Texas group, said at a news conference.
Both research groups halted blastoid development at day 10, shy of the 14-day end of the International Association for Stem Cell Research which honors the formation of the primitive streak, which is thought to be marking the beginning of the development of the nervous system. The group is considering lowering that limit.
Both variations on the blastoid subject matter are not just like bona fide human blastocysts, said Amander Clark, PhD, of the University of California, Los Angeles, which is part of the iBlastoids team. “They are organized, embryo-like structures, shaped on human embryos, but I don’t consider them the same as human blastocysts that come from IVF [in vitro fertilization] clinics. “Blastoids contain some cells that are not in blastocysts and can be cell culture contaminants.
“Blastoid technology is likely to catalyze further research that will lead to a better understanding of early human development, which is something of a black box,” Paul Knoepfler, PhD, professor in the Department of Cell Biology and Human Anatomy at the University of California, Davis, School of Medicine, Sacramento, California Medscape Medical News.
Since the most recent embryos are mice and the earliest are not true replicas of their human contemporaries, they can, for now, consider practical applications to eliminate anxiety. biochemical.
“While blastoids can only model those few early days in human development, those days are critical to our overall development,” said Polo, of Monash University. “For example, we would be able to understand infertility, because we know that a large proportion of miscarriages occur in the first weeks of pregnancy. We can study congenital complications and diseases from the beginning and study the effects of drugs, toxins, and viruses in the early stages of development, all without the use of human or animal embryos. “
Knoepfler connects the dots located in the three studies. “The new method for developing mouse embryos to mid-gestation in non-maternal filters will enhance our knowledge of mammalian development in general. This technology could also be used in theory to make human embryos by IVF or human blastoids in filters in the lab. “
But these abilities raise the biological questions.
“For example, would laboratory – made human embryos have a different status than those that underwent routine IVF or reproduction? What about embryos that started IVF, but then grown in a vial? in a lab instead of a mother? ” asked Knoepfler.
Nature. Published online 17 March 2021. Study by Hanna and colleagues, Abstract; Study by Polo and colleagues, Abstract; Study by Wu and colleagues, Abstract
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.