Editor’s note: Discover the latest COVID-19 news and instructions in the Medscape Coronavirus Resource Center.
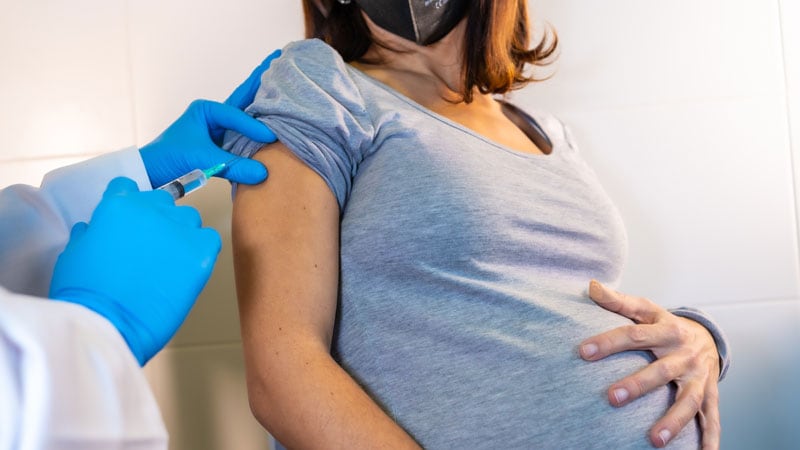
COVID-19 vaccines should not be withheld from pregnant or lactating persons who wish to receive the vaccine, despite the lack of safety data in these numbers, according to guidance from the -Centres for Disease Control and Prevention (CDC), American College of Obstetricians and Gynecologists (ACOG), and the Society for Maternal-Fetal Medicine.
Pregnant women who do not choose to be vaccinated should also be supported in that decision, a practice adviser from ACOG suggests.
“Pregnant women with fever should be counseled after taking the acetaminophen vaccine,” the counseling notes.
In addition, women do not need to become pregnant after receiving the Pfizer-BioNTech COVID-19 vaccine, according to the CDC’s interim clinical consideration for its use. The U.S. Food and Drug Administration issued an emergency use permit (EUA) for the vaccine on Dec. 11.
Although researchers have banned pregnant women from clinical trials, experts believe that mRNA vaccines, which are not live vaccines, are “unlikely to pose a risk to pregnant women” and ” they are considered a danger to the breastfeeding baby, “the CDC notes.
At the same time, pregnant women may be at increased risk of COVID-19 infection, even though the overall risk of serious illness is low. COVID-19 may also increase the risk of adverse pregnancy outcomes, such as premature birth, although the data have been mixed with some studies finding association and others not.
“If pregnant women are part of a group that is recommended to have the COVID-19 vaccine (eg, health care workers), they can opt to have the vaccine,” the CDC advises. ” Discussions between the patient and their clinical team may help with decisions regarding the use of vaccines agreed under the EUA for the prevention of COVID-19. While talking to a healthcare provider may be helpful, it is not necessary before you have the vaccine. “
Recognizing side effects and uncertainties
ACOG counseling replicates that approach. The group notes that, based on the mechanism of action of the mRNA vaccine and its safety and efficacy in clinical trials, “the safety and efficacy profile of the vaccine for pregnant women is expected to be similar. to those seen in infertile people … That said, there are no specific safety data regarding the use of mRNA vaccine in pregnant or lactating people and the potential risks are unknown. for a pregnant woman and the fetus. ”
In clinical trials, most participants experienced flu-like symptoms after receiving the vaccine, including injection site reactions, muscle weakness, colds, muscle and joint pain , and a salty head. Among participants aged 18–55 years, fever above 38 ° C occurred in 3.7% of participants after the first dose and in 15.8% after the second dose. Resolve most tokens within a few days.
Pregnant women with fever should be treated with acetaminophen because “fever has been associated with adverse fetal outcomes,” according to ACOG guidelines. “Acetaminophen has been shown to be safe for use in pregnancy and does not appear to affect the antibody response to COVID-19 vaccines.” Patients can be treated with other vaccine side effects, such as injection site pain with acetaminophen also.
When counseling patients, clinicians should explain that side effects are a normal part of developing antibodies to protect against COVID-19. “Whatever their decision,” the group says, “these conversations allow patients to be reminded of the importance of other preventative measures such as hand washing, body speed, and wear a mask. “
More data expected
Data from developmental and reproductive toxicity studies in animals are expected soon, the CDC said. In addition, the manufacturer is following clinical trial participants who became pregnant throughout the study.
Pregnant women and their physicians should emphasize factors such as the rate of COVID-19 transmission in the community, the patient’s risk of contracting COVID-19, the risks of COVID-19 to the patient and the patient. fetus, vaccine efficacy and side effects, and lack of COVID-19 vaccine data during pregnancy.
The Society for Maternal Fetal Medicine recommends that pregnant or lactating women generally have access to COVID-19 vaccines and has advocated for pregnant or lactating women in vaccine trials. The association has recommended that health care professionals “advise their patients that the theoretical risk for fetal damage from mRNA vaccines is very low.” It published resources this week for physicians and patients with a focus on COVID-19 vaccine and pregnancy.
In a study published online December 10 in the American Journal of Obstetrics & Gynecology MFM, Amanda M. Craig, MD, of Duke University Health System in Durham, North Carolina, and coauthors note that there is a “theoretical risk for fetal damage from any unproven medical intervention and this is no different for COVID-19 vaccines. “
“Pregnant people, along with their obstetric provider, should be given the opportunity to minimize the potential risk of severe maternal disease against an unknown risk of fetal exposure, and make an autonomous decision about whether to accept the vaccine until fertile safety data is available. , “they write.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube.