
Ro

The use of modern electrolyte will allow advanced metal electrodes and higher voltages, enhancing cycling capacity and life.
Lithium-ion batteries have enabled the lightweight electronic devices that carry what we now take for granted, as well as a rapid expansion in electric vehicle production. But researchers around the world are continuing to push the boundaries of ever-increasing energy density – the energy that can be stored in a certain amount of material – to achieve the performance of existing devices. already promote and to enable new applications such as far. -range drones and robots.
One promising way is to use metal electrodes instead of the conventional graphite, with a higher cost voltage in the cathode. These efforts were thwarted, however, by a combination of unwanted chemical reactions with the electrolyte that separates the electrons. Now, a team of researchers at MIT and elsewhere modern electrolyte has overcome these problems and could enable a huge jump in the power-per-weight of next-generation batteries, without sacrificing cycling life.
The research is reported in the journal The Power of Nature in a paper by MIT professors Ju Li, Yang Shao-Horn, and Jeremiah Johnson; postdoc Weijiang Xue; and another 19 at MIT, two national laboratories, and elsewhere. The researchers say this discovery could enable lithium-ion batteries, which typically typically store around 260 watts-hours per kilogram, to store about 420 watts-hours per kilogram. . That would translate into longer areas for electric cars and longer modifications to portable appliances.
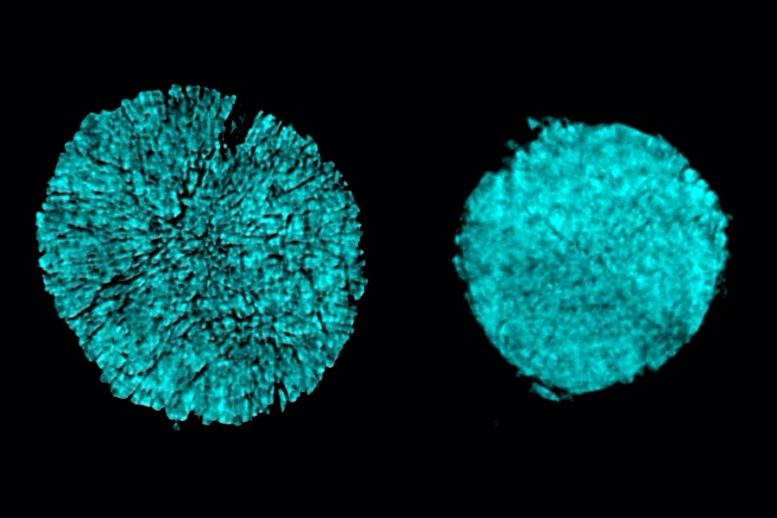
X-ray tomography images taken at Brookhaven National Lab show a splitting of several in one electrode of a battery cell that used a conventional electrolyte (as seen on the left). The researchers found that novel electrolyte use prevented most of this cracking (right). Credit: By permission of the researchers
The basic raw materials for this electrolyte are cheap (although one of the intermediate fertilizers is still expensive because it is used sparingly), and the process for making it is simple. Therefore, this improvement could be implemented relatively quickly, the researchers say.
The electrolyte itself is not new, explained Johnson, a professor of chemistry. It was developed a few years ago by some members of this research team, but for a different application. It was part of an effort to develop lithium-ion batteries, which are seen as the ultimate long-term solution for increasing battery energy density. But there are still many obstacles to the development of such batteries, and the technology may still be years away. In the meantime, adding that electrolyte to lithium-ion batteries with metal electrodes turns out to be something that can be accomplished much faster.
The new application of this electronic material was discovered “something serendipitously,” after it was first developed a few years ago by Shao-Horn, Johnson, and others, in a collaborative venture aimed at on lithium-air battery development.
“There is still nothing that will allow a good lithium-air battery to be recycled,” Johnson says. However, “we designed those organic molecules that we hoped would provide stability, compared to the soluble electrolytes used. ”They developed three different formulas based on sulfonamide, which they found to be highly resistant to oxidation and other polluting effects. Then, working with Li’s group, postdoc Xue decided to try this material with more custom cathodes.
The type of battery electricity they now use is this electrolyte, nickel oxide which contains some cobalt and manganese, “this is the work of today’s electric vehicle industry,” says Li , who is a professor of nuclear science and engineering and materials science and engineering.
As the electrical material expands and contracts anisotropically as it is charged and dissipated, this can lead to cracking and breakdown in performance when used with conventional electrolytes. But in experiments in collaboration with Brookhaven National Laboratory, the researchers found that using the new electrolyte significantly reduced these pollution levels in terms of pressure.
The problem was that the metal atoms in the alloy prone to melting into the molten electrolyte, losing mass and leading to cracking of the metal. In contrast, the new electrolyte is strongly resistant to such a dispersion. Looking at the data from the Brookhaven tests, Li says, it was “surprising to see that if you change the electrolyte, those cracks are gone. ”They found that the morphology of the electrolyte material is much stronger, and that the transition metals are not“ just as flexible ”in these new electrolytes.
That was an amazing combination, he says, because the material still allows lithium ions to pass through – the essential way batteries charge and discharge – while charging. preventing the other cations, called moving metals, from entering. The accumulation of unwanted compounds on the surface of the electrolyte after many dispersion-reduction cycles was reduced by more than tenfold compared to the conventional electrolyte.
“The electrolyte is chemically resistant to the oxidation of nickel-filled high-energy materials, prevents particle breakage and stabilizes the advanced electricity during cycling,” says Shao-Horn, professor of mechanical engineering and materials science. and engineering. “The electrolyte also enables permanent strip and plating of lithium metal, an important step towards enabling rechargeable lithium-metal batteries with energy twice the energy of modern lithium-ion batteries. This finding leads to further research and electrolyte design of molten electrolytes for lithium-metal batteries competing with those with solid state electrolytes. ”
The next step is to scale the production so that it is affordable. “We do it in one very easy response from readily available commercial starter products,” Johnson says. At the moment, the precursor material used to synthesize the electrolyte is expensive, but he says, “I think if we can show the world that this is a good electrolyte for consumer electronics, the incentive to scale more will help drive the price down. ”
Since this is an “insert” instead of an electrolyte and does not require a redesign of the entire battery system, Li says, it could be activated quickly and could be commercialized within a year or two. “There are no expensive elements, just carbon and fluorine. So it’s not limited by resources, it’s just the process, ”he says.
Details: “Ni-rich ultra-voltage molten cathodes in practical sulfonamide-based electrolyte-enabled Li metal batteries” by Weijiang Xue, Mingjun Huang, Yutao Li, Yun Guang Zhu, Rui Gao, Xianghui Xiao, Wenxu Zhang, Sipei Li, Guiyin Xu, Yang Yu, Peng Li, Jeffrey Lopez, Daiwei Yu, Yanhao Dong, Weiwei Fan, Zhe Shi, Rui Xiong, Cheng-Jun Sun, Inhui Hwang, Wah-Keat Lee, Yang Shao-Horn, Jeremiah A. Johnson and Ju Li, The Power of Nature.
DOI: 10.1038 / s41560-021-00792-y
The research was supported by the U.S. Department of Energy and the National Science Foundation, and used facilities at Brookhaven National Laboratory and Argonne National Laboratory.