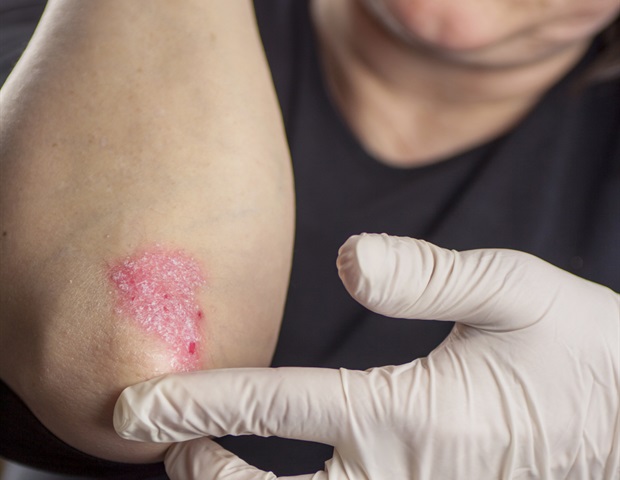
Psoriasis is a chronic infectious skin disease characterized by erythematous patches and plaques (red). In some patients, psoriasis may be associated with comorbidities such as arthritis, obesity, diabetes, cardiovascular disease, hyperlipidemia, or depression. Psoriasis injuries can occur on the scalp and face, as well as all other parts of the body, causing physical discomfort and psychosocial trauma from the stigma surrounding appearance defects.
More than 90% of patients with psoriasis are called psoriasis vulgaris, which is characterized by plaques, while the other types have less than 5%: arthritic psoriasis, pustular psoriasis, and erythrodermic psoriasis. The most common treatments for moderate to severe plaque psoriasis are standard corticosteroids and vitamin D3 analogs.
However, long-term use of these conventional steroids often causes various side effects such as skin atrophy, hypo- or hyper-pigmentation, telangiectasia, and skin diseases. And long-term use of vitamin D3 analogs may increase the need for interventions to regulate calcium and phosphate metabolism. These effects on the body are very important and require routine and systemic treatments for psoriasis.
In a study recently published in Chinese Medical Journal, scientists from prestigious research centers and hospitals across China, led by Professor Jian-Zhong Zhang from Peking University Population Hospital, have partially met this need. Benvitimod, a modern contemporary non-steroidal drug, has been reported to be effective and safe for the treatment of plaque psoriasis.
Speaking about their research trip, Prof. Zhang says, “Benvitimod (also known as tapinarof) is a small molecule that was first isolated from the bacterium Photorhabdus luminescens and was synthesized by the late Genhui Chen and his team from Beijing Wenfeng Tianji Pharma. He took more than 20 years to study the mechanism of action, come up with the design, and evaluate the effectiveness and safety of this cement in patients with psoriasis. It was really hard work. “
In their study, Professor Zhang and his colleagues conducted a double-blind, placebo-controlled phase III clinical trial in twenty-three dermatological centers in China to evaluate the safety and efficacy of benvitimod co-surfactants. topical in patients with moderate-to-severe psoriasis plaque.
Six hundred and eighty-six adult patients with moderate to severe plaque psoriasis were included in this study. Patients were randomized to receive a 1% benvitimod cream, 0.005% calcipotriol ointment – a standard treatment for plaque psoriasis – or “placebo.” They were treated for 12 weeks. The efficacy of benvitimod was evaluated at the end of the treatment period.
Of the patients treated with benvitimod cream, 50.4% achieved a psoriasis area score and a severity index (PASI) of 75 at 12 weeks, indicating a 75% or greater reduction in size and severity. depth of psoriasis compared to pretreatment levels. In comparison, PASI 75 was achieved in only 38.5% and 13.9% of patients receiving calcipotriol ointment and placebo, respectively. Similarly, a significantly higher proportion of patients who received benvitimod cream (66.3%) achieved a global physician static assessment score (sPGA) of 0 or 1 (indicating severe disease severity) compared to those who was placed on placebo (33.5%).
This phase III test also showed that the benvitimod surface was well tolerated. The most common side effects were mild discomfort and itch at the site of application and these symptoms were resolved independently in the majority of patients. No systemic adverse effects were detected.
The results of this phase III test are encouraging. The benvitimod cream may soon be a new treatment option for Chinese patients with plaque psoriasis. As most patients with psoriasis have plaque psoriasis, this will have a significant impact on reducing the burden of psoriasis in the country. “
Professor Jian-Zhong Zhang, Peking University Population Hospital
Perhaps, over time, it can become the norm in the management of psoriasis worldwide.
Source:
Magazine Reference:
Cai, L., et al. (2020) A double-blind, randomized, placebo- and placebo-controlled phase III trial of a 1% benvitimod cream in moderate-to-severe plaque psoriasis. Chinese Medical Journal. doi.org/10.1097/CM9.00000000000000122121.