
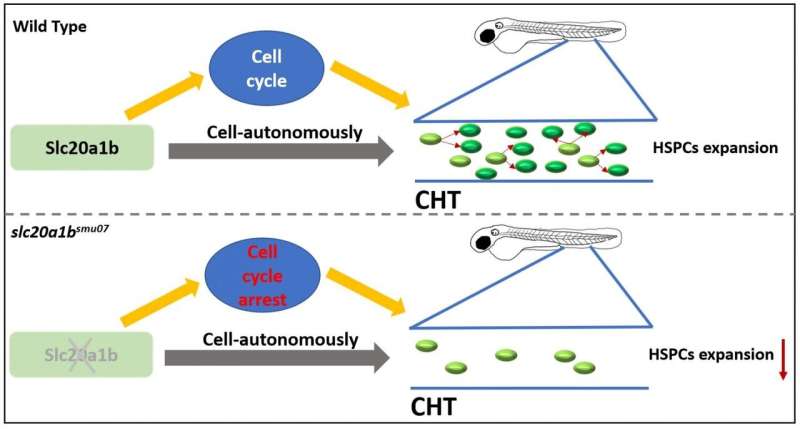
The working model of this research Credit: Science China Press
Hematopoietic gas / progenitor cells (HSPCs) consist of hematopoietic stem cells and a number of biased hematopoietic progenitor cells, which can secrete all types of blood cells in an adult organism. Among them, hematopoietic gas cells have the ability to self-renew and multiply differentiate, and can respond rapidly under hungry hematopoietic conditions. At the same time, the hematopoietic progenitor cells can maintain a supply of blood cells in homeostatic hematopoiesis. In a word, HSPCs are the heart of the blood system, once their homeostasis is destroyed, it will lead to serious blood diseases and even death. Thus, the studies associated with the HSPCs can provide strong support for relevant theoretical studies and clinical therapies.
To study the development of the hematopoietic system, researchers often use zebrafish as a model, which has the benefits of a short growth cycle, many visible eggs, in vitro fertilization of embryos, easy access to gene preparation, and so on. In addition, the hematopoietic system of zebrafish is closely linked to the human system, which is also the basis for the application of zebrafish in hematopoietic studies.
Back in 2012, Wenqing Zhang research group and Yiyue Zhang research group collaborated to advance genetic screening of zebrafish, introduce random gene mutations in zebrafish through ENU compound, and obtain a series of mutants zebrafish with hematopoietic deficiencies. Based on this research, the collaborative team continues to study these mutants, digging deeper into the suppressed genes and through the ways in which these genes cause deficiencies. in the hematopoietic system.
Recently, a new research paper of Yiyue Zhang Laboratory and Wenqing Zhang Laboratory, named as “Slc20a1b essential for hematopoietic gas cell / progenitor expansion”, was published in Life Sciences China Science. This study showed that point mutation of zebrafish slc20a1b gene can significantly reduce the growth of HSPCs, leading to severe hematopoietic development.
In this research, the researchers first performed a deeper phenotypic identification of the smu07 mutant, obtained with the previous ENU mutagenesis. The hematopoietic defects of the erythroid, myeloid, and lymphoid lines of the mutant were all present, suggesting that the upstream HSPCs may have been damaged. Further study showed that the mutant HSPCs could be excreted normally at the initial stages, but then failed to proliferate continuously, and the number of HSPCs was significantly lower than the normal ones. number of typical zebrafish.
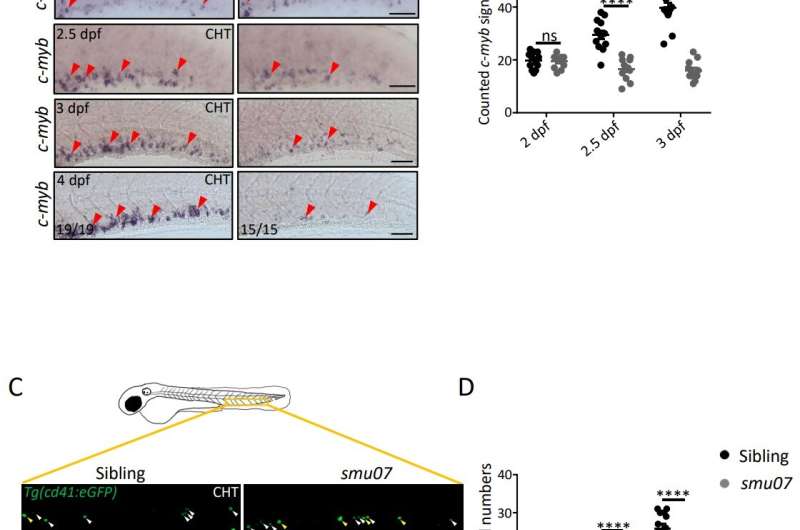
The HSPCs of smu07 mutant lacked expansion capabilities. Credit: © Science China Press
Next, the researchers genetically mapped the mutation site and identified the causative gene as slc20a1b, in which the mutation of a single DNA base caused a mutation of one amino acid in its protein (D48E ). With the help of CRISPR-Cas9 technology, they constructed an additional slc20a1b mutant, which was a similar phenotype to the smu07 mutant, confirming that the gene causing the imitation was slc20a1b.
Because slc20a1b is a widely expressed gene, the researchers further investigated whether genetic mutations in HSPCs were responsible for its phenotype, or whether other cells played a key role in its location. . They found that the mutant HSPCs were unable to expand in a normal environment, while the wild-type HSPCs were able to expand in the mutant environment. This result confirmed that the defect was caused by autonomic mutation of the slc20a1b gene in HSPCs.
Finally, the researchers examined the cell mechanism of the HSPC-deficient HSPCs and found that the cell death of mutant HSPCs was unchanged, but a defect of its proliferative ability prevented the HSPCs from proliferating. The cell cycle of mutant HSPCs was blocked in the G2 / M phase and therefore could not complete the normal proliferation cycles.
In summary, the authors found that slc20a1b mutant HSPCs automatically developed an enlargement defect, which is manifested by cell cycle arrest leading to reduced proliferation (Figure 2), leading to severe deficiency of HSPCs and all hematopoietic lines.
This study is the first to report the essential role of the slc20a1b gene in HSPCs, revealing a modern regulatory gene of the HSPCs cell cycle.
How bone marrow regenerates after chemotherapy
Jiakui Chen et al, Slc20a1b is essential for hematopoietic stem / progenitor cell proliferation in zebrafish, Life Sciences China Science (2021). DOI: 10.1007 / s11427-020-1878-8
Presented by Science China Press
Citation: Slc20a1b essential for hematopoietic gas / progenitor cell proliferation in zebrafish (2021, March 26) retrieved March 26, 2021 from https://phys.org/news/2021-03-slc20a1b-essential-hematopoietic-stemprogenitor- cell.html
This document is subject to copyright. Other than any fair treatment for the purpose of scrutiny or private investigation, no part may be reproduced without written permission. The content is provided for informational purposes only.