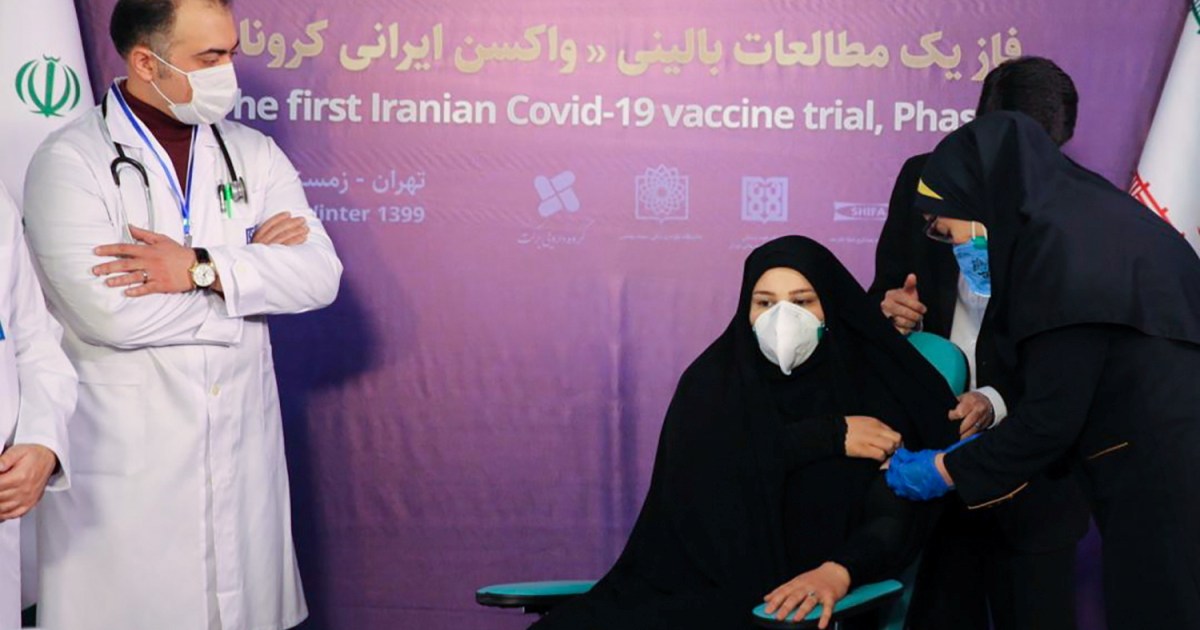
The announcement comes on the same day that Iran’s first vaccine, COVIran Barekat, completed the first phase of human testing.
Tehran, Iran – A second locally made COVID-19 vaccine will begin human trials in Iran, officials said.
The vaccine, called Razi COV-Pars, was unveiled Sunday at a ceremony in Tehran attended by several senior officials.
It was produced by the Vaccine Research Institute and Serum Razi, the oldest vaccination institute in Iran with a history of almost 100 years.
According to the study by the Iranian Food and Drug Administration, Kianoush Jahanpour, the vaccine will be tested on 130 volunteers in its first phase.
He said Razi COV-Pars is an mRNA vaccine that reproduces a harmless piece of the virus’s spike protein. It is evaluated in both injectable and inhalation form in its first phase, with the results determining the end-use protocol.
Health minister Saeed Namaki told the audience at the opening ceremony that the vaccine has so far seen very few side effects and will stop the person who gets the virus from passing it on to others.
Agriculture minister Kazem Khavazi said animal testing of the vaccine began more than nine months ago and tested on about 500 animals.
“Testing on 25 monkeys was also an unprecedented record and a huge task when a large number of Razi institute workers caught the virus,” he was quoted as saying by an IRNA run with the state.
The news came on the same day that Iran’s first vaccine, COVIran Barekat, had completed the first phase of human trials that saw the vaccine administered to 56 volunteers, including several officers.
Human trials for the local two-dose vaccine began in late December and officials have said the early results will be published in less than a month.
Hojjat Niki Maleki, head of media for Setad, the powerful state-led body under the Supreme Leader Khamenei that runs the Barekat project, said on Sunday that two Arab countries have officially asked to buy the vaccine without naming the countries.
Meanwhile, the first shipment of 10,000 doses of Russia’s Sputnik V vaccine reached Iran last week just over a week after Iran’s foreign minister Mohammad Javad Zarif announced in Moscow that the vaccine approved for emergency use in Iran.
Sputnik V, which bought a purchase on a vaccine debate in Iran, is said to be 91.6 percent effective in peer-reviewed results published last week.
The first doses of the vaccine are slated to be given to health care professionals working in intensive care units in Iranian hospitals Tuesday, one day before the 42nd anniversary of the birth of Iran ‘s current regulatory system.
Iran is fighting the deadliest outbreak in the Middle East of COVID-19, with more than 58,000 lives lost and more than 1.4 million confirmed diseases.