According to the CDC, traumatic brain injury (TBI) is a leading cause of death and disability in the US. Each year approximately 1.5 million Americans maintain TBI. From 2006 to 2014, the number of TBI-related emergency department visits, hospitals, and deaths increased by 53% .1 According to the U.S. Department of Defense, up to 75% of armed forces reported TBI visual changes.2
Based on these data, approximately 1.1 million Americans with TBI can visit the offices of eye care practitioners each year reporting significant changes in quality of life and needing support with activities of daily living.
Several types of visual disorders are common after the effects of TBI. Inflammatory injuries, penetrative injuries, and blunt injuries may cause structural damage to the eye or lesions or inflammation of the brain that may interfere with visual pathways.3 In contact with patients and patient care teams, it is important to help non-eye care providers that most of the sequelae to TBI are not related to ocular but related to changes in the brain and the pathways that control it. on the eyes.
Between 2000 and early 2016, more than 347,962 TBIs were diagnosed in the U.S. armed forces, with about 80% classified as mild TBIs (mTBI) .3
In a study to determine the prevalence of vision disorders after mTBI, 100 patients were surveyed, and 69% had 1% or more of the following vision studies:
– Accommodation Disorders (51%)
– Incompatibility of assembly (49%)
– Saccadic deprivation (29%)
Overall, 46% of patients received more than 1 vision diagnosis.4 For a review of visual changes in moderate to severe brain injury including loss of visual field, see part 1 of t. this series (“Visual Rehabilitation of Stroke Patients,” November 2020).
Most mTBI symptoms resolve after 28 days.5 If a patient is severely anxious, the National Vision Center of Excellence provides an evidence-based care algorithm for ophthalmology and optometry in patients who reports visual changes after mTBI.3 These patients often require 2 visits for the clinician to collect all relevant data to provide optometric visual acuity. For the primary care clinician, the following protocol is helpful.
Test protocol
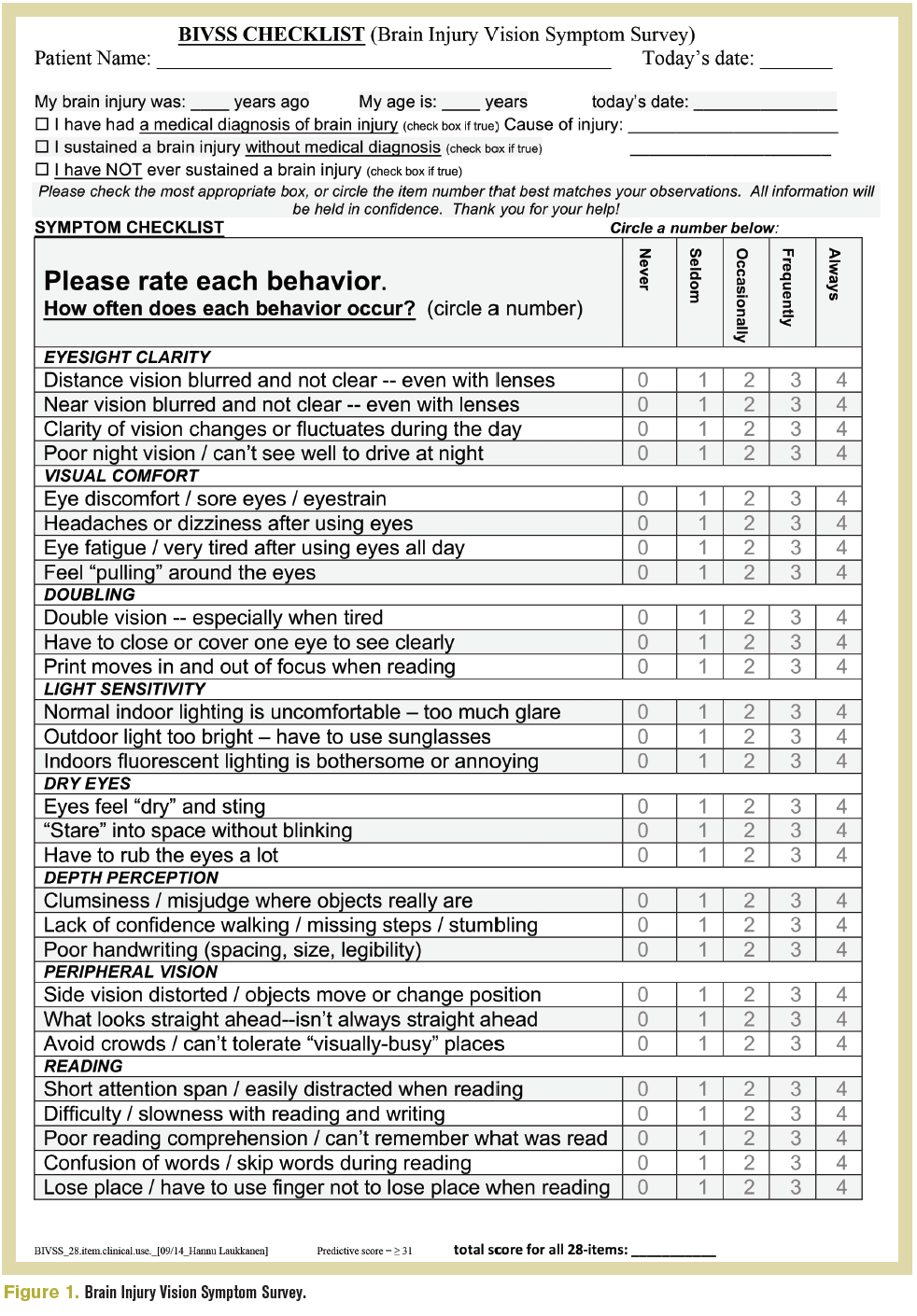
A comprehensive eye examination is the basis for a detailed action vision assessment, all starting with a detailed case history. The Brain Injury Vision Symptom Study (Figure 1) can guide the new or inexperienced clinician on the types of questions that may be asked.
Optimal visual clarity should be measured through traditional methods. Dynamic visual acuity should compare visual acuity while the head is moving relative to the static baseline acuity. This assessment examines the ability of the vestibular ocular reflex (VOR) to maintain a stable vision during head movement, known as gaze establishment.
To achieve, use the best corrected visual acuity as the minimum line, and present 4 layers of accuracy above it. If 20/20 is the best correction acuity, the Snellen chart should show 20/50 going down to 20/20. As they look at the card, ask the patient to move their head side to side (approximately 20˚ left and right at centerline) at a frequency of 2 side-by-side circles per second and read to the lowest possible line. A decrease in acuity to 2 lines worse than baseline indicates a defect in the responsiveness of the VOR.
Computer visual fields should be performed to control ischemic or stressful events along the traditional visual path. Careful anterior and posterior assessment will reassure the clinician that all visual complaints are related to visual cortical acuity.
To measure binocular function, start with a cover test at and near speed. While the patient is looking at the target with good posture and head position, ask the patient if the target is moving sideways, up and down, or diagonally. This may alert the clinician to direct small heterophoria that may cause severe symptoms.
While performing a torso and saccade assessment, ask the patient if eye movement is causing symptoms and note hypermetric or hypometric action.
In addition to traditional near-concentration (NPC) assessment, a red lens NPC test can detect small deficiencies in sensory fusion. To perform, ask the patient to wear red / green glasses above their normal range near correction and use a penlight. Starting outside the range of separation marked on the traditional NPC, move the penlight inwards until diplopia is reported, and repeat 3 times. Diplopia at 7 cm or longer is a failure.
A small study showed that 76% of mTBI sufferers complained of photophobia.6 Evidence also shows that mTBI sufferers have a lower incidence of emergency cough, 7 indicating that the number of patients this is more sensitive to the frequency at which transient light stimuli appear to be completely constant. . Therefore, due to the amount of screen time at this time, mTBI patients are often more comfortable wearing the highest level of ophthalmic grade blue-lens lenses. These are a tool that is readily available in the arsenal of the primary care clinician that will bring great relief.
Look for a cure
Approximately 28 days or more after injury, the presence of reduced visual acuity, a cover test outside of clinical norms and / or with the presence of direct heterophoria, symptoms during eye movements, and / or reduced NPC indicate transmission to comorbidity. a worker capable of offering optometric vision.
Working with patients who have suffered from TBI is a beneficial part of optometric care. For more information on how you should work with this patient population, visit https://visionhelp.com/concussion/ or www.noravisionrehab.com.
References
1. TBI: Get the facts. CDC. Updated March 11, 2019. Accessed February 28, 2021. https://www.cdc.gov/traumaticbraininjury/ get_the_facts.html
2. Goodrich GL, Kirby J, Cockerham G, et al. Visual function in polytrauma rehabilitation center patients: a descriptive study. J Reset Res Dev. 2007; 44 (7): 929-36. doi: 10.1682 / jrrd.2007.01.0003
3. Eye and vision care follows the manifestation of a possible explosion and / or traumatic brain injury. Lèirsinn Center of Excellence. Updated 24 November 2015. Accessed 28 February 2021. https: // vce. health.mil/Products/Additional-Resources/TBI-CR-Photo
4. Master CL, Scheiman M, Gallaway M, et al. Visual diagnosis is common after decision making in adolescents. Clin Pediatr (Phila). 2016; 55 (3): 260-267. doi: 10.1177 / 0009922815594367
5. McCrory P, Meeuwisse W, Dvořák J, Divine J. Consensus statement on decision-making in sport – 5th international conference on decision-making in sport held in Berlin, October 2016. Br J Sports Med. 2017; 51: 838-847. doi: 10.1136 / bjsports-2017-097699
6. Clark J, Hasselfeld K, Bigsby K, et al. Stained glasses to relieve the symptoms of posttraumatic brain injury photophobia. Train J Athl. 2017; 52 (8): 725-729. doi: 10.4085 / 1062-6050-52.4.04
7. Chang TTL, Ciuffreda KJ, Kapoor N. Frequency of emergency filter and associated symptoms in mild traumatic brain injury. Brain Inj. 2007; 21 (10): 1055-1062. doi: 10.1080 / 02699050701591437